|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
Mice retinal cells regenerated Earlier research from Reh's lab observed in newborn mice that MG cells can be stimulated to become retinal neurons by activating a particular protein which controls transcription of DNA into mRNA. A factor called Ascl1 activates a suite of genes involved in regeneration. However, by the time baby mice become adults, regions of their genetic code are shut down and the Ascl1 transcription factor becomes inaccessible. After screening a library of small molecules to find one that might make the adult genetic code accessible again, Reh's team found it in a genetically engineered mouse — one molecule that reopens Ascl1 in MG cells. 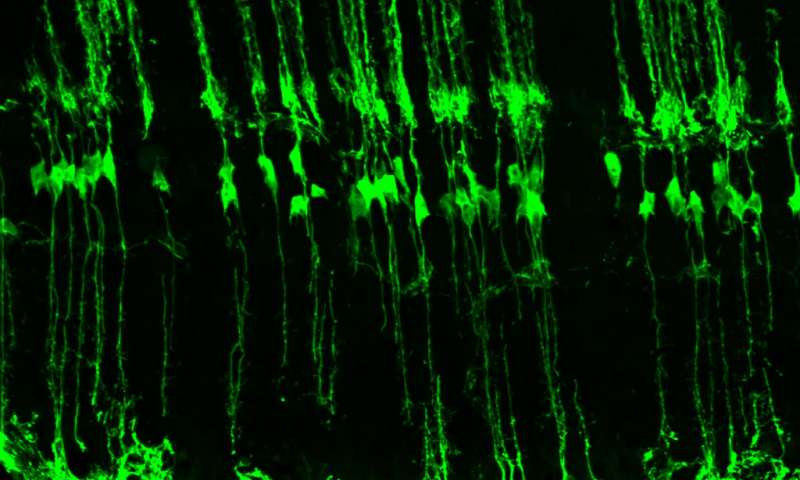 Glia are supportive cells in the retina. In zebrafish they undergo changes when the retina is injured, turning into a variety of cells to repair the damage. Credit: Tom Reh/University of Washington. After injuring adult mouse retinas with a toxin, researchers injected the mice with TSA and tamoxifen. Over the next several weeks, the shape and behavior of the fluorescent green-labeled cells revealed evidence of regeneration. "We found that the commonly used anti-cancer agent trichostatin A (TSA) made critical regions of DNA accessible again. Ascl1 could then bind to those regions, which stimulated the regeneration of neurons in adult mice," explained Nikolas Jorstad, post doctoral student in the Reh lab. Analyses of the restored cells revealed their genome structure had been MG, but now genetically reprogrammed, showed characteristics of interneurons. About two weeks following injury, looking at the cells' electrophysiological patterns, revealed them to be responding as interneurons. "We're showing for the first time that Müller glia in the adult mouse can give rise to new neurons after injury. And, these neurons have the gene expression pattern, the morphology, the electrophysiology and the epigenetic program to look like interneurons instead of glia." The new cells formed functioning synapses - connections from one neuron to another - and respond to light in a way typical of interneurons and are also integrated with retinal cells that convey signals to the brain. Reh envisions his approach as useful for treatment of acute eye injuries and central retinal arterial occlusion - or stroke in the eye. The next step will be to boost MG numbers."Retinal injuries and blinding diseases of the retina tend to cause a massive loss of neurons. We need a way to stimulate the regeneration of Müller glia (MG) cells, in addition to strategies for coaxing them to differentiate into other types of neurons," says Reh. Strategies are also needed to regenerate photoreceptors and ganglion cells, and other types of retinal cells lost in degenerative eye diseases such as glaucoma and macular degeneration. Reh's lab is investigating other regenerative strategies to address all retinal cell types. Abstract Many retinal diseases lead to the loss of retinal neurons and cause visual impairment. The adult mammalian retina has little capacity for regeneration. By contrast, teleost fish functionally regenerate their retina following injury, and Müller glia (MG) are the source of regenerated neurons. The proneural transcription factor Ascl1 is upregulated in MG after retinal damage in zebrafish and is necessary for regeneration. Although Ascl1 is not expressed in mammalian MG after injury, forced expression of Ascl1 in mouse MG induces a neurogenic state in vitro and in vivo after NMDA (N-methyl-D-aspartate) damage in young mice. However, by postnatal day 16, mouse MG lose neurogenic capacity, despite Ascl1 overexpression. Loss of neurogenic capacity in mature MG is accompanied by reduced chromatin accessibility, suggesting that epigenetic factors limit regeneration. Here we show that MG-specific overexpression of Ascl1, together with a histone deacetylase inhibitor, enables adult mice to generate neurons from MG after retinal injury. The MG-derived neurons express markers of inner retinal neurons, synapse with host retinal neurons, and respond to light. Using an assay for transposase-accessible chromatin with high-throughput sequencing (ATAC–seq), we show that the histone deacetylase inhibitor promotes accessibility at key gene loci in the MG, and allows more effective reprogramming. Our results thus provide a new approach for the treatment of blinding retinal diseases. Keywords: Regeneration and repair in the nervous system Neurogenesis Reference: Jorstad, NL., et al. 2017. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature DOI: 10.1038/nature23283 NEI leads the federal government's research on the visual system and eye diseases. NEI supports basic and clinical science programs to develop sight-saving treatments and address special needs of people with vision loss. For more information, visit https://www.nei.nih.gov. About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit http://www.nih.gov/. Return to top of page |
Aug 30, 2017 Fetal Timeline Maternal Timeline News News Archive 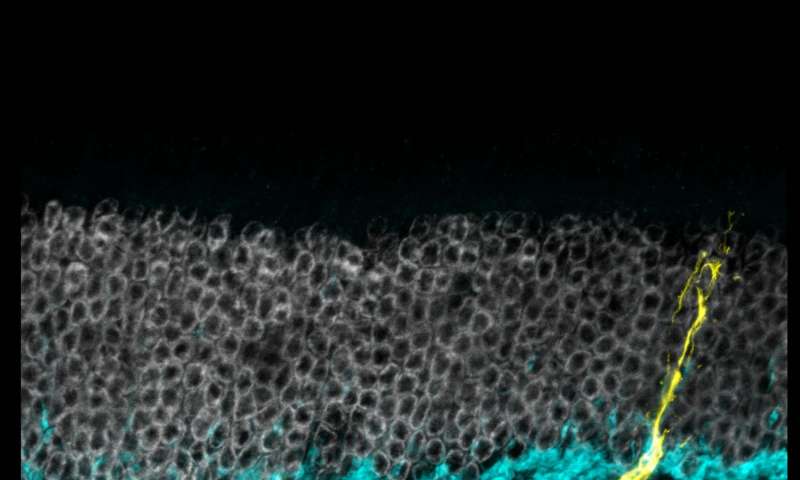 A microscope image showing glia cells and neurons in the eye's retina. Image credit: Tom Reh lab/University of Washington Medicine
|
||||||||||||||||||||||||||||

