|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
Nerves control our body's bacterial community A central aspect of biology is the symbiotic relationships which exist between animals, plants and humans. Each with their own specific bacterial communities. Scientists refer to the full set of microorganisms living on and inside a host organism as its microbiome. Over the past years, evidence has accumulated that the composition and balance of an individual's microbiome contributes to that organism's health. For example, alterations in composition of these bacterial communities are implicated in originating various so-called environmental diseases. However, it is still largely unknown just how the cooperation between organism and bacteria works at the molecular level and how the microbiome and body exactly act as a functional unit. 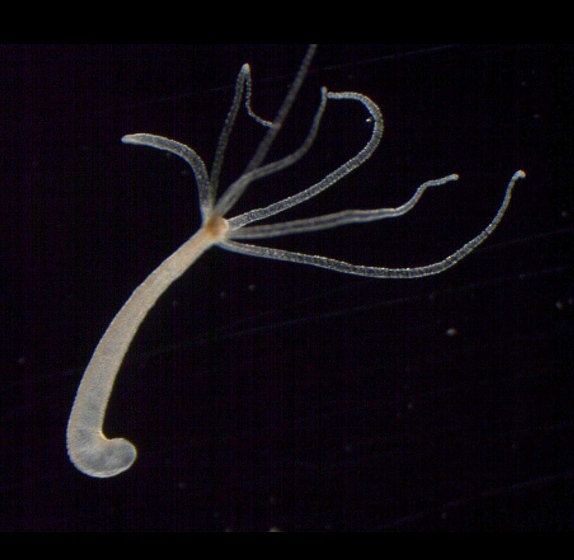 The freshwater polyp 'Hydra'. An important breakthrough in deciphering these highly complex relationships has now been achieved by a research team from Kiel University Zoological Institute in Germany. Using the freshwater polyp Hydra as their model organism, Kiel researchers and their international colleagues, investigated how the simple nervous system of these ancient animals interacts with its own microbiome. Researchers demonstrate that small molecules secreted by hydra nerve cells help regulate the composition of colonies of beneficial bacteria along a Hydra's columnar body. "Up to now, neuronal factors that influence the body's bacterial colonization were largely unknown. We have proved that the nervous system plays an important regulatory role here," explains Professor Thomas Bosch, evolutionary developmental biologist at the Collaborative Research Center's 'Origin and Function of Metaorganisms.' The scientists published their findings in Nature Communications. Bosch leads the research of freshwater polyps as a model organism. Hydra are an ancient branch of the animal kingdom, with a simple body plan and only about 3000 neurons in their neural net. Despite this simplicity, they share molecular similarity with the nervous systems of all vertebrates, enabling our deeper appreciation of ancient, fundamental principles of how the nervous system was built to function. Using the hydra, researchers collected cellular, molecular and genetic evidence showing how neuropeptides antibacterial activity affects the composition and spatial distribution of colonizing microbes. Researchers were able to concentrate on development of the nervous system from egg to adult animal as Cnidarians develop a complete nervous system in under three weeks. During this time, bacterial communities covering the animal's surface change radically, until a stable composition of the microbiome finally forms. Under the antimicrobial affect of neuropeptides, the concentration of Gram-positive bacteria (a subgroup) decreases sharply at about four weeks. At the end of this maturing process, typical composition is dominated by Gram-negative Curvibacter bacteria. As neuropeptides are produced in only certain areas of the body, they also control the spatial localization of bacteria along the body column. For example, in the head region there is a strong concentration of antimicrobial peptides, resulting in six times fewer Curvibacter bacteria on the head than on Hydra tentacles. Based on these observations, the scientists conclude that throughout evolution, the nervous system participated in a controlling role for the Hydra microbiome, in addition to exercising sensory and motor tasks. "The findings are also important in an evolutionary context. Since the ancestors of these animals invented the 'nervous system,' it seems the interaction between the nervous system and the microbiome is an ancient feature of multicellular animals. Abstract Colonization of body epithelial surfaces within a highly specific, microbial community is fundamental to all animals. Yet, the underlying mechanisms by which these communities are selected and maintained is not well understood. Here, we show that sensory and ganglion neurons in the ectodermal epithelium of the Hydra (a member of the animal phylum Cnidaria) secretes neuropeptides with antibacterial activity. These may shape the microbiome on the Hydra body surface. In particular, a specific neuropeptide, which we call NDA-1, contributes to the reduction of Gram-positive bacteria during early development and thus to spatial distribution of the main colonizer, the Gram-negative Curvibacter sp., along the body axis. Our findings warrant further research to test whether neuropeptides secreted by nerve cells contribute to the spatial structure of microbial communities in other organisms. Contributors René Augustin, Katja Schröder, Andrea P. Murillo Rincón, Sebastian Fraune, Friederike Anton-Erxleben, Ava-Maria Herbst, Jörg Wittlieb, Martin Schwentner, Joachim Grötzinger, Trudy M. Wassenaar, Thomas C.G. Bosch (2017): "A secreted antibacterial neuropeptide shapes the microbiome of Hydra". Nature Communications, Published on September 26, 2017, doi:10.1038/s41467-017-00625-1 R.A. and K.S. were responsible for the experimental design and contributed equally to prepare the manuscript. R.A. performed protein purification, MIC assays, qRT-PCR, and (with K.S.) immunocytochemistry. K.S. generated the constructs for transgenesis and recombinant expression in E. coli (with E.-M.H.), performed in situ hybridization and mechanical stimulation. A.P.M.R. performed qRT-PCR and the GSH assay. F.A.-E. was responsible for confocal microscopy. M.S. performed the phylogenetic analysis. J.G. was responsible for the structural modeling of NDA-1. T.M.W. assisted in graphical presentation of the data and writing the manuscript. T.G.C.B. supervised the project and was the driving force behind the research concept. Competing interests The authors declare no competing financial interests. Return to top of page |
Oct 4, 2017 Fetal Timeline Maternal Timeline News News Archive  surround (green) nerve cells.png) Fibres of intestinal tissue (in red) surround the nerve cells (in green) of the freshwater polyp Hydra. Image Credit: Christoph Giez, Dr. Alexander Klimovich, Kiel University Zoological Institute, Germany
|
||||||||||||||||||||||||||||

