|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
Deveopmental biology Kidney organoids test drugs A new, open-source and free database, is now available at the GenitoUrinary Development Molecular Anatomy Project (GUDMAP), providing the first systematic, high-resolution atlas (databank) for the genesis of human kidneys. "Stem-cell based technologies hold great promise for developing kidney replacement and regeneration therapies," explains Nils Lindstrom, first author on three new studies. Lindstrom is a research associate in Stem Cell Biology and Regenerative Medicine at the Keck School of Medicine, University of Southern California (USC). "Getting there requires detailed knowledge of how kidneys normally form so the process can be replicated in cell cultures in the lab. Our data will help us and other scientists improve current techniques to make better tiny functional kidneys." The median wait time for a kidney transplant is 3.6 years, during which patients must go to three dialysis appointments per week. Over the past four years, USC Stem Cell researchers and USC Viterbi School of Engineering computer scientists have documented molecular, cellular and genetic similarities and differences between human and mouse kidney formation so they can find treatments for kidney disease affecting approximately 30 million or 15 percent of US adults. Published Feb. 15, 2018 in the Journal of the American Society of Nephrology, the three-study series provides the first cellular and molecular study of how the human kidney develops in a mother's womb. "Our research bridges a critical gap between animal models and human applications. The data we collected and analyzed creates a knowledge-base that will accelerate stem cell-based technologies to produce mini-kidneys that accurately represent human kidneys for biomedical screening and replacement therapies," says Jill A. McMahon, whose lab has focused on the kidney for the past 25 years. How human and mouse kidneys are similar The kidney plays a central role in controlling the body's ecosystem by regulating blood pressure and removing waste products. Nephrons are the smallest functional unit of the kidney that help remove blood waste from the body. The form only during fetal life, before the stem cells that generate them are exhausted. Researchers analyzed developmental differences in timing and basic structure between human and mouse kidneys to gain insight into the regulatory processes maintaining and expanding kidney stem cells into mature, functional kidneys. Researchers compared 26 human kidney "anchor genes" with their mouse equivalents. Anchor genes are required for organ development, but only three genes have comparable expression between mouse and human kidneys: SLC22A6, ENTPD5 and UMOD. "If the goal is to treat human kidney disease, clearly, it's better to focus on genes that are also active in human kidneys," McMahon adds. An Intractable Problem Kidney disease is a big, intractable problem, requiring a multidisciplinary approach. So, McMahon teamed up with Carl Kesselman, study co-author and Dean's Professor of Industrial and Systems Engineering at USC Viterbi. Kesselman's team built software to automate tasks such as recording data from high-resolution microscopes. This not only fast-tracked one of the three studies, it also created an online, searchable library to help other stem cell scientists in kidney disease research. "If you think of data as the modern version of a book, we gave researcher tools to write the book, made the library where the book is stored and created a catalog system so others can find the book and check it out," explains Kesselman, a principal investigator at the USC Michelson Center for Convergent Bioscience. The result is the Discovery Environment for Relational Information and Versioned Assets or DERIVA, a virtual photo album easily shared with other researchers. Abstract Human kidney function is underpinned by approximately 1,000,000 nephrons, although the number varies substantially, and low nephron number is linked to disease. Human kidney development initiates around 4 weeks of gestation and ends around 34–37 weeks of gestation. Over this period, a reiterative inductive process establishes the nephron complement. Studies have provided insightful anatomic descriptions of human kidney development, but the limited histologic views are not readily accessible to a broad audience. In this first paper in a series providing comprehensive insight into human kidney formation, we examined human kidney development in 135 anonymously donated human kidney specimens. We documented kidney development at a macroscopic and cellular level through histologic analysis, RNA in situ hybridization, immunofluorescence studies, and transcriptional profiling, contrasting human development (4–23 weeks) with mouse development at selected stages (embryonic day 15.5 and postnatal day 2). The high-resolution histologic interactive atlas of human kidney organogenesis generated can be viewed at the GUDMAP database (www.gudmap.org) together with three-dimensional reconstructions of key components of the data herein. At the anatomic level, human and mouse kidney development differ in timing, scale, and global features such as lobe formation and progenitor niche organization. The data also highlight differences in molecular and cellular features, including the expression and cellular distribution of anchor gene markers used to identify key cell types in mouse kidney studies. These data will facilitate and inform in vitro efforts to generate human kidney structures and comparative functional analyses across mammalian species. Authors: Nils O. Lindström1, Jill A. McMahon1, Jinjin Guo1, Tracy Tran1, Qiuyu Guo1, Elisabeth Rutledge1, Riana K. Parvez1, Gohar Saribekyan1, Robert E. Schuler2, Christopher Liao1, Albert D. Kim1, Ahmed Abdelhalim1, Seth W. Ruffins1, Matthew E. Thornton3, Laurence Basking4, Brendan Grubbs3, Carl Kesselman2,5 and Andrew P. McMahon1 Jill McMahon, Jinjin Guo, Tracy Tran, Qiuyu Guo, Elisabeth Rutledge, Riana Parvez, Gohar Saribekyan, Christopher Liao, Albert Kim, Ahmed Abdelhalim, Seth Ruffins and Andrew Ransick from the Department of Stem Cell Biology and Regenerative Medicine at the Keck School of Medicine; Robert Schuler from the Information Sciences Institute at USC Viterbi; Matthew Thornton, Brendan Grubbs, Guilherme De Sena Brandine and Andrew D. Smith from USC; and Laurence Basking from the University of California, San Francisco also contributed to this study. About USC Stem Cell USC Stem Cell is a collaborative, multidisciplinary effort working to translate the potential of stem cell research to the clinical imperative of regenerative medicine. Centered at the Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at USC, the initiative brings together nearly 100 research and clinical faculty members from the Keck School of Medicine of USC, Children's Hospital of Los Angeles, the USC Viterbi School of Engineering, the USC Davis School of Gerontology, the Ostrow School of Dentistry of USC, the USC School of Pharmacy, and the USC Dornsife College of Letters, Arts and Sciences. About USC Michelson Center for Convergent Bioscience The USC Michelson Center for Convergent Bioscience, located in Michelson Hall, brings together a diverse network of premier scientists and engineers from the USC Dornsife College of Letters, Arts and Sciences, USC Viterbi School of Engineering and Keck School of Medicine of USC to solve some of the greatest intractable problems of the 21st century - from cancer, to neurological disease, to cardiovascular disease. With a generous $50 million gift from Gary K. Michelson, a retired orthopedic spinal surgeon, and his wife, Alya Michelson, the USC Michelson Center for Convergent Bioscience occupies the largest building on campus, a state-of-the-art facility for USC to transform and influence the course of scientific discovery and biomedicine for generations to come. Information about the USC Michelson Center for Convergent Bioscience is available at https://michelson.usc.edu/. The research was supported by the California Institute for Regenerative Medicine (CIRM LA1-06536), National Institutes of Health (NIH DK107350, DK094526, DK110792, 5F32DK109616-02), National Institute of Diabetes and Digestive and Kidney Diseases (F31DK107216, 1U24DK110814) and Joint Medical/Research Council/Wellcome Trust (099175/Z/12/Z) as well as by a USC Stem Cell Hearst Fellowship and a USC Research Enhancement Fellowship. Return to top of page | Feb 19, 2018 Fetal Timeline Maternal Timeline News News Archive 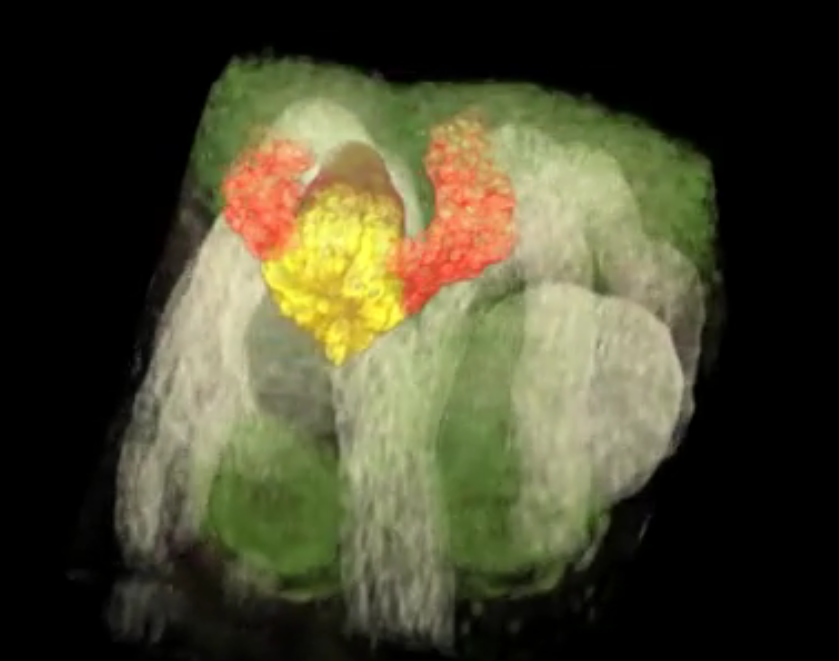 The Kidney Organoid (YELLOW) initiates development of other kidney structures (LIGHT GREEN) seen emerging from the organoid. These images were captured in real time and in 3D. Image credit: Video courtesy of Nils Lindstorm, Andy McMahon, Seth Ruffins, the Microscopy Core Facility, Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research, Keck School of Medicine of USC
|
||||||||||||||||||||||||||||

