|
|
Developmental biology - Genome
'Jumping genes' are critical to early embryo
'Jumping genes' make up one-fifth of our human genome and are key to the first stages of embryo cell division...
So-called "jumping genes" researchers previously (and for a long time) considered either genetic junk or a pernicious parasite turn out to critically regulate the first stages of embryo development, according to the University of California San Francisco scientists. The work is published in Cell.
Only about 1 percent of the human genome encodes proteins, and researchers have long debated what the other 99 percent is good for. Many of these non-protein coding regions are known to contain important regulatory elements that orchestrate gene activity, but others are thought to be evolutionary garbage that is just too much trouble for the genome to clean up.
Fully half of our DNA is made up of "transposable elements" or "transposons" virus-like genetic material that has the special ability of duplicating and reinserting itself in different locations in the genome, which has led researchers to dub them genetic parasites.
Over the course of evolution, some transposons have left hundreds or thousands of copies of themselves scattered across the genome. While most of these are thought to be inert and inactive, others alter or disrupt cells' normal genetic programming and have been associated with diseases such as certain forms of cancer.
Now UCSF scientists find the most common transposon, LINE1, accounting for 20 percent or more of the human genome, is actually necessary for embryos to develop past the two-cell stage.
'Playing with Fire'
Study senior author Miguel Ramalho-Santos PhD, associate professor of obstetrics/gynecology and reproductive sciences and a member of the Eli and Edythe Broad Center for Regeneration Medicine and Stem Cell Research at UCSF, has been interested in transposons since he became an independent UCSF Fellow in 2003.
He had observed that embryonic stem cells and early embryos both expressed high levels of LINE1, paradoxical for a gene at that time considered to be a dangerous, disease-causing parasite. "Given the standard view of transposons, these early embryos were really playing with fire. It just didn't make any sense, and I wondered if something else was going on."
The project took off when Michelle Percharde PhD, joined his lab as a post-doctoral researcher in 2013. She was quickly caught up in Ramalho-Santos's enthusiasm for the LINE1 paradox. Percharde: "When I saw all the LINE1 RNA that's present in the nucleus of developing cells, I agreed there must be some role it's playing. Why let your cells make so much of this RNA if it's either dangerous or doing nothing?"
To determine whether high levels of LINE1 RNA expression in mouse embryos are actually important to an animals' development, Percharde eliminated LINE1 RNA from mouse embryonic stem cells.
To her surprise, the pattern of gene expression in these cells changed, reverting to a pattern seen in (1) two-cell embryos following fertilization and (2) at the egg's first division.
Without LINE1 from fertilized eggs, embryos completely lost their ability to progress past the two-cell phase.
"Protein-coding genes...are less than 2 percent of the genome, whereas transposons make up nearly 50 percent. When we saw that cells were changing identity when we removed LINE1 RNA, that was our real 'Aha!' moment that told us we were on to something," says Percharde.
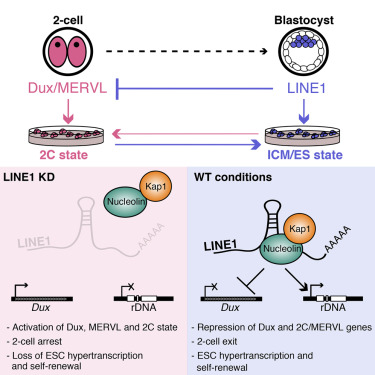
Further experiments revealed that although the LINE1 gene is expressed in the early embryo and stem cells, its role is not to insert itself elsewhere in the genome. Instead, its RNA is trapped within the cell nucleus, in a complex with 2 gene regulatory proteins: (1) Nucleolin and (2) Kap1. This complex is needed to turn off the dominant gene program orchestrating an embryos' two-cell state - controlled by a gene called Dux - and to turn on genes needed for the embryo to continue generating cell divisions for its development.
Duplication May Add Robustness
The research took five years and Percharde's invention of several procedures to study transposons, but resulted in a finding they hope will convince other scientists to finally pay attention to the functional roles of jumping genes.
"These genes have been with us for billions of years and have been the majority of our genomes for hundreds of millions of years. I think it's fair to ask if the 1.5 percent of protein-coding genes are the real free-riders, and not the other way around."
Miguel Ramalho-Santos PhD, Associate Professor, Obstetrics and Gynecology and Reproductive Sciences, member of the Eli and Edythe Broad Center for Regeneration Medicine and Stem Cell Research at UCSF.
Ramalho-Santos speculates that transposons like LINE1 may make the delicate early stages of development more robust precisely because they are so pervasive. Because LINE1 is repeated thousands of times in the genome, it's virtually impossible for a mutation to disrupt its function: if one copy is bad, there are thousands more to take its place.
Ramalho-Santos: "We now think these early embryos in a very calculated way, could be a very robust mechanism for regulating development."
Highlights
LINE1 RNA is abundant and nuclear in mouse ESCs and pre-implantation embryos
LINE1 knockdown inhibits ESC self-renewal and induces transition to a 2C state
LINE1 RNA recruits Nucleolin/Kap1 to repress Dux and activate rRNA synthesis
In embryos, LINE1 inhibition causes persistence of the 2C program and impairs ZGA
Summary
Transposable elements represent nearly half of mammalian genomes and are generally described as parasites, or junk DNA. The LINE1 retrotransposon is the most abundant class and is thought to be deleterious for cells, yet it is paradoxically highly expressed during early development. Here, we report that LINE1 plays essential roles in mouse embryonic stem cells (ESCs) and pre-implantation embryos. In ESCs, LINE1 acts as a nuclear RNA scaffold that recruits Nucleolin and Kap1/Trim28 to repress Dux, the master activator of a transcriptional program specific to the 2-cell embryo. In parallel, LINE1 RNA mediates binding of Nucleolin and Kap1 to rDNA, promoting rRNA synthesis and ESC self-renewal. In embryos, LINE1 RNA is required for Dux silencing, synthesis of rRNA, and exit from the 2-cell stage. The results reveal an essential partnership between LINE1 RNA, Nucleolin, Kap1, and peri-nucleolar chromatin in the regulation of transcription, developmental potency, and ESC self-renewal.
Authors: Michelle Percharde, Chih-Jen Lin, Yafei Yin, Juan Guan, Gabriel A. Peixoto, Aydan Bulut-Karslioglu6, Steffen Biechele, Bo Huang, Xiaohua Shen and Miguel Ramalho-Santos.
The authors declare no competing interests.
Return to top of page
| |
|
Jul 2, 2018 Fetal Timeline Maternal Timeline News News Archive
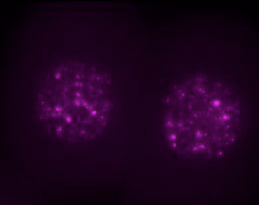 Above, single two-cell mouse embryos with nuclear LINE1 RNA (MAGENTA). Image credit: Ramalho-Santos Lab.
|