|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
Developmental biology - Breathing How Breathing Fails to Work In Preemies and SIDS The expression, 'As easy as breathing' is truly misleading. Breathing in higher vertebrates is complex. It is a biological act involving many types of neurons: chemo-sensory neurons to sense oxygen and carbon dioxide levels in blood; motor neurons to control muscle movement; and specialized neurons that establish breathing rhythms at birth and maintain them throughout life. The mechanism orchestrating all of these diverse groups of neurons has been a mystery. But now, Dr. Huda Zoghbi and her colleagues have found two neuronal lines in our hindbrain that act as 'conductors' for this exquisite biological symphony. They coordinate the relay of sensory information from chemo-sensors in our blood to rhythmogenic neurons in our central respiratory circuit. They establish and maintain optimal breathing rhythms critical to the survival of a newborn. "The ability to modulate respiratory rhythms in response to changes in the levels of oxygen and carbon dioxide is crucial to the survival of all animals. During fetal development, most hardwired neural circuits practice fine-tuning their activities based on feedback they receive in utero." At birth, these neuronal circuits must be precisely wired and ready to perform flawlessly under challenging circumstances — should oxygen levels become low at high altitude, or during exercise, and when carbon dioxide builds up during chronic obstructive pulmonary disease, or with asthma, and during sleep apnea. Neural circuits must quickly make breaths deeper and faster. "Understanding how these key genes set up the breathing circuit could provide valuable insights into how the neural respiratory circuits are 'jumpstarted' at birth," said Zoghbi, also an investigator at the Howard Hughes Medical Institute. "Moreover, it also could offer key insights into the cause of respiratory distress in premature babies and infants who die of sudden infant death syndrome (SIDS)." Master regulatory genes oversee development of neuronal circuits and coordinate responses between them. These genes crisscross our entire hindbrain, forming a checkerboard pattern, being the genetic blueprint on which our neural respiratory circuit is built. Atoh1 (Atonal homolog 1) is one of those regulatory genes. For decades, scientists saw mice without Atoh1 die of respiratory failure at birth. Expressed along the entire length of the hindbrain, it is essential to accurate development of several neural lineages in the hindbrain. A previous study conducted by Zoghbi's lab saw when Atoh1 is deleted from the retrotrapezoid nucleus (RTN) - neurons located in the lower part of the hindbrain - mice couldn't increase their respiratory rhythm in response to an increase in carbon dioxide. Yet, only some newborn mice in such litters died. This suggests other Atoh1 expressing neurons play a role in breathing. Zoghbi was motivated to study other Atoh1-lineage neurons. Using genetic lineage tracing techniques, Meike van der Heijden, a graduate student in the lab and primary author on the paper, found parabrachial complex neurons are specifically activated when oxygen and carbon dioxide levels change. This suggests they may play an important role in regulating breathing under stress. "Atoh1 was specifically deleted from the upper rhombic lip region, part of the upper hindbrain where PBC neurons are born. Although mice lacking parabrachial complex neurons survived the early neonatal period, they exhibited several hallmarks of immature respiratory control such as rhythmic irregularities and apneas. Also, these mice were unable to breathe faster or deeper when oxygen was low. Together, this suggests parabrachial complex neurons are required for proper maturation of the respiratory network." Researchers next asked how parabrachial complex neurons communicate sensory information to the central respiratory circuit. Do they activate downstream rhythmogenic neurons or act directly on muscles? Using a newly generated mouse line that distinguishes Atoh1-lineages from other hindbrain neurons, they conclude parabrachial complex neurons act indirectly by communicating with rhythmogenic neurons and RTN neurons. The authors also show that abnormal development of parabrachial complex or RTN neurons repeat aspects of immature breathing control found in premature infants. Although these neuronal lines respond to different sensory cues, both appear to be critical to initiating faster breathing rhythms. Mice with a combined loss of Atoh1 in both the parabrachial complex and RTN neurons had severe breathing problems and died at birth. This shows that although the two cell types may somewhat compensate for loss of one another, they must act together to ensure survival. 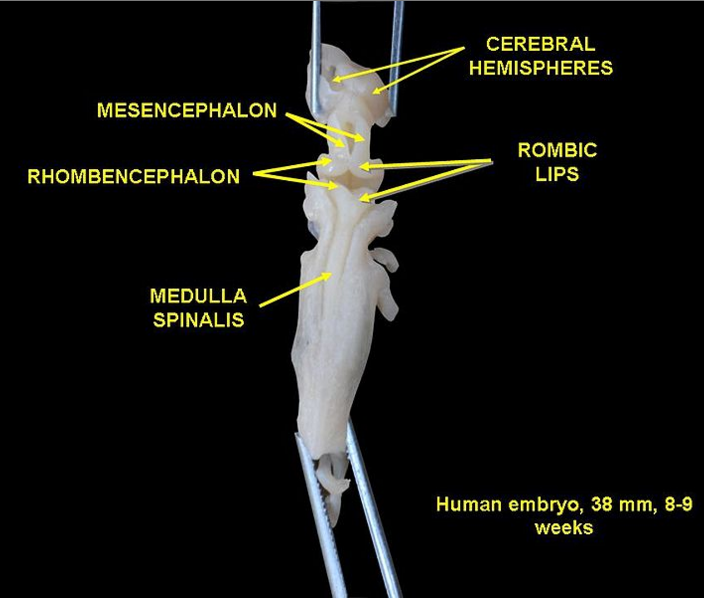 Human embryo 8 - 9 weeks. Credit: Wikimedia Based on their results, the authors propose that changes in oxygen and carbon dioxide levels - at birth or due to high altitude and exercise - are detected by chemosensory neurons and transmitted to (1) the parabrachial complex and (2) RTN neurons. RTN neurons then relay this data to rhythmogenic neurons, activating motor neurons and muscles which allow an animal to take quick, deep breaths and inhale more oxygen. The study also offers excellent molecular and cellular targets for future investigations to pinpoint how dysfunctional Atoh1 lineages might contribute to respiratory failure in premature and high-risk babies. Understanding what are the key nodes in the respiratory network critical to robust breathing, is key in physicians understanding breathing vulnerabilities in newborns and preventing SIDS-related fatalities in the future. Read all the details of this work in the journal eLife. Abstract Atoh1-null mice die at birth from respiratory failure, but the precise cause has remained elusive. Loss of Atoh1 from various components of the respiratory circuitry (e.g. the retrotrapezoid nucleus (RTN)) has so far produced at most 50% neonatal lethality. To identify other Atoh1-lineage neurons that contribute to postnatal survival, we examined parabrachial complex neurons derived from the rostral rhombic lip (rRL) and found that they are activated during respiratory chemochallenges. Atoh1-deletion from the rRL does not affect survival, but causes apneas and respiratory depression during hypoxia, likely due to loss of projections to the preBötzinger Complex and RTN. Atoh1 thus promotes the development of the neural circuits governing hypoxic (rRL) and hypercapnic (RTN) chemoresponses, and combined loss of Atoh1 from these regions causes fully penetrant neonatal lethality. This work underscores the importance of modulating respiratory rhythms in response to chemosensory information during early postnatal life. Authors Meike E van der Heijden and Huda Y Zoghbi. Funding This project was supported by the Neurobehavioral Core facility (U54HD083092), the Neuroconnectivity Core facility (U54HD083092), and the RNA In Situ Hybridization Core facility (with the expert assistance of Cecilia Ljungberg, Ph.D., 1S10 OD016167 and IDDRC grant 1U54 HD083092, Eunice Kennedy Shriver National Institute Of Child Health and Human Development). This work also was supported by American Heart Association AWRP Predoctoral Fellowship 17PRE33660616. Return to top of page | Sep 13, 2018 Fetal Timeline Maternal Timeline News News Archive  A new study analyzes the influence of weight and height on lung development and asthma risk in nearly 4,500 children in Rotterdam (Netherlands). Photo by Charlein Gracia.
|
||||||||||||||||||||||||||||