|
|
Developmental biology - Organ Development
Controlling Organ Growth With Light
Optogenetics can make structural changes in cells...
In optogenetics, researchers are using light to control protein activity. The technique allows them to either inhibit or make alterations in the shape of embryonic tissue - and observe resulting developmental abnormalities. Scientists in the European Molecular Biology Laboratory (EMBL) led by Sterano De Renzis, have now enhanced their technique so that it stops organ-shaping processes in fruit fly embryos. The research is published in EMBO Journal, revealing how they control crucial steps in embryo development.
For healthy development, cells have to change shape. Abnormalities in the shape of cell surface transitions can lead to problems in organ growth.
For example, when groups of cells alter their shape to make organs, the De Renzis team observed the mechanics behind these transitions using light to affect cell surface flexibility.
To form internal organs, groups of cells must move to the inside of an embryo, a process called invagination. During invagination the surface of the cell contracts to pull it inward and allow it to fold. Abnormalities in this process lead to problems in tissue and organ development.
"Imagine the embryo as a balloon and tissue invagination as the deformation caused by fingers pushing the surface of the balloon inwards. The only difference is that cells are not subjected to external force like fingers, but need to generate forces to move inside by themselves."
Stefano De Renzis PhD, European Molecular Biology Laboratory, Heidelberg, Germany and project leader.
Initiating and inhibiting invagination
By inhibiting the naturally occurring invagination of cells, De Renzis' group now better understands what drives the process. Crucial to invagination is flexibility in the tissue surface allowing it to fold inwards. By using optogenetics to stiffen the cell surface, it cannot bend inwards, stopping the whole process. "To stick with the balloon analogy, when you simultaneously squeeze the top and the bottom of a balloon, the inner pressure increases and the balloon can't fold inwards anymore," explains De Renzis.
With optics, it is not only possible to stop invagination before it begins, but to stop it midway. The EMBL team feels it can now modify protein activity without damaging cells - activating and deactivating cell modifications as needed. Combined with their previous results, they are able to control every step of embryo cell surface invagination.
Their results are the first proof of a long-standing theory explaining morphological abnormality in embryo development. Although initial experiments were done in fruit fly embryos, De Renzis expects these methods can be applied to other organisms. Potentially, they want to create and shape artificial tissues and alter tissue development for regenerative medicine.
Abstract
Tissue invagination drives embryo remodeling and assembly of internal organs during animal development. While the role of actomyosin-mediated apical constriction in initiating inward folding is well established, computational models suggest relaxation of the basal surface as an aditional requirement. However, the lack of genetic mutations interfering specifically with basal relaxation has made it difficult to test its requirement during invagination so far. Here we use optogenetics to quantitatively control myosin-II levels at the basal surface of invaginating cells during Drosophilia gastrulation. We show that while basal myosin-II is lost progressively during ventral furrow formation, optogenetics allows the maintenance of pre-invagination levels over time. Quantitative imaging demonstrates that bending slows down cell elongation and blocks invagination. Activation after cell elongation and tissue bending has initiated inhibits cell shortening and folding of the furrow into a tube-like structure. Collectively, these data demonstrate the requirement of myosin-II polarization and basal relaxation throughout the entire invagination process.
Authors
Daniel Krueger, Pietro Tardivo, Congtin Nguyen and Stefano De Renzis.
Acknowledgements
The authors thank E. Izquierdo, A. Reversi, and all members of the De Renzis laboratory for helpful discussion. We thank U. Schwarz and A. Degen for critical reading of the manuscript. We thank the advanced light microscopy core facility (European Molecular Biology Laboratory (EMBL), Heidelberg) for their advice and assistance, and A. Politi for providing the Pipeline Constructor macro. We thank the Bloomington Drosophila Stock Center for providing fly stocks and the Drosophila Genomics Resource Center for providing cDNAs.
Funding
European Molecular Biology Laboratory (EMBL)
Return to top of page
| |
|
Nov 28, 2018 Fetal Timeline Maternal Timeline News News Archive
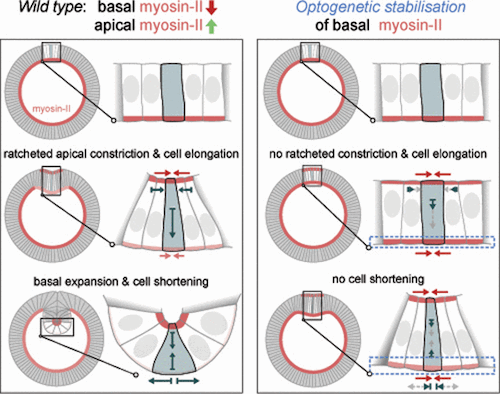 Slice through (top to bottom) view of invagination process. As the outer or apical surface of a cell contracts, the inner or basal surface relaxes. This coordinated process generates force driving the cell towards the inside of the embryo - which is the first step towards development of an organ. VIDEO Credit: Daniel Krueger
|



