|
|
Developmental Biology - Olfactory System
Gender Affects Sense of Smell
Olfactory receptors in mice change when exposed to odors from members of the opposite sex...
A new study is investigating how mouse olfactory sensory receptors change when exposed to odors emitted by the opposite sex. The work was done by Stephen Santoro PhD, and assistant professor, Department of Zoology and Physiology, University of Wyoming. The number of olfactory receptors increases with exposure to more odor stimulation.
"The idea is that our experiences change our sensory system in a way that is semipermanent. This is probably true in humans as much as mice," Santoro says. "Mice are a very good model to understand how neural systems work, in general. They are a much better model for humans than flies and other common-model organisms."
"We found mice housed with the opposite sex all of the time have olfactory sensory receptors similar in composition because they are smelling similar smells. On the other hand, mice housed separately by sex have sex-specific differences in their olfactory receptors."
Stephen W. Santoro PhD, Neuroscience Program, Department of Zoology & Physiology, University of Wyoming, Wyoming, USA
Mouse odors are a complex mixture of volatile and non-volatile chemicals derived from skin secretions, urine, tears, saliva and feces, which are known to differ substantially in their chemical compositions between males and females. The study is published in Nature Communications.
Sensory activity is pivotal to development of the nervous system. However, unlike most neurons in the mammalian nervous system, olfactory sensory neurons (OSNs) are continually born and replaced throughout life. Of interest to science.
The same process replaces damaged neurons in humans when we have a cold or use a zinc nasal spray. Increases in the number of specific OSN subtypes, such as the vomero nasal organ (VNO) — mainly used to detect pheromones carrying messages between males and females of the same species — partly occurs through a use-it-or-lose-it mechanism. Under stimulated and numbers of OSNs and VNOs decline.
"As we age, our olfactory system gets worse. One of NIH's priorities is to understand why that occurs. We think our research may provide insights into why olfactory neurons die and how the birth of new neurons is regulated."
Stephen W. Santoro PhD
Abstract
Within the mammalian olfactory sensory epithelium, experience-dependent changes in the rate of neuronal turnover can alter the relative abundance of neurons expressing specific chemoreceptors. Here we investigate how the mouse olfactory sensory receptor repertoire changes as a function of exposure to odors emitted from members of the opposite sex, which are highly complex and sexually dimorphic. Upon housing mice either sex-separated or sex-combined until six months of age, we find that sex-separated mice exhibit significantly more numerous differentially expressed genes within their olfactory epithelia. A subset of these chemoreceptors exhibit altered expression frequencies following both sex-separation and olfactory deprivation. We show that several of these receptors detect either male or female specific odors. We conclude that the distinct odor experiences of sex-separated male and female mice induce sex-specific differences in the abundance of neurons that detect sexually dimorphic odors.
Authors
Carl van der Linden, Susanne Jakob, Pooja Gupta, Catherine Dulac and Stephen W. Santoro.
Acknowledgements
Neuroscience Program, Department of Zoology & Physiology, University of Wyoming, 1000 E. University Avenue, Laramie, WY, 82071, USA
Carl van der Linden, Pooja Gupta & Stephen W. Santoro
The research was funded by the National Institutes of Health (NIH), including through a grant to the Wyoming Sensory Biology Center of Biomedical Research Excellence, and by the Howard Hughes Medical Institute in Chevy Chase, Md. Results from this study may contribute to an enhanced understanding of sex-specific differences in olfactory function.
Howard Hughes Medical Institute, Department of Molecular and Cellular Biology, Center for Brain Science, Harvard University, 16 Divinity Avenue, Cambridge, MA, 02138, USA
Susanne Jakob & Catherine Dulac
Return to top of page
| |
|
Dec 21, 2018 Fetal Timeline Maternal Timeline News News Archive
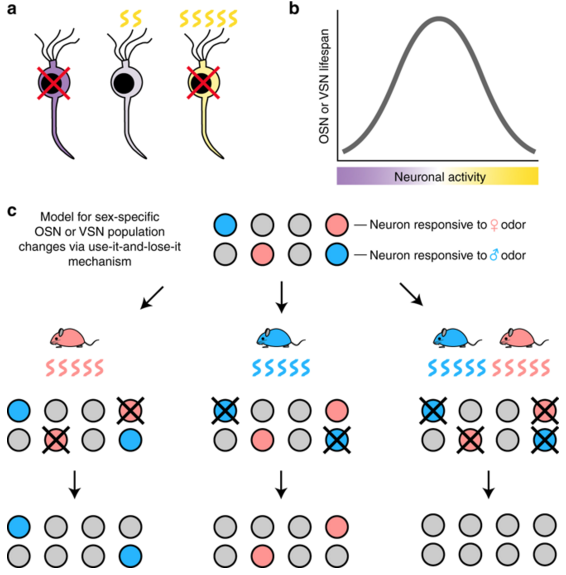 Mouse model for how OSN and VSN subtypes become differentially abundant: (a) Very low (VIOLET) or very high (YELLOW) activity may shorten OSN/VSN lifespan; a moderate (GRAY) level of activity may lengthen lifespan (b) An optimal range of neuronal activity may maximize OSN/VSN lifespan.
|



