|
|
Developmental Biology - Sperm Stem Cells
How sperm stem cells maintain high numbers
To continually replenish sperm in the testis, mice rely on Fibroblast Growth Factors...
The steady production of sperm relies on the number of sperm stem cells in the testis remaining constant. In tissues like the testis and ovary of the fruitfly Drosophila and intestine of mammalians, stem cells are clustered in specialized niches where self-renewal-promoting factors are abundant and called stem cell niches. In these tissues, stem cell numbers are controlled simply by the capacity of the niche. However, sperm stem cells are not clustered in mouse testis, but are highly motile and widely dispersed across the basement membrane — or outer (basal) surface of the seminiferous tubes — which are embedded with myoid (muscle-like) cells.
These myoid cells contract to help move sperm cells and fluid through the seminiferous tubes. Researchers found that a subset of lymphatic endothelial (LE) cells produce Fibroblast Growth factors or FGFs (in particular: Fgf5, 8 and 4), which promote stem cell self-renewal.
"Lymphatic endothelial cells were identified in the testis via electron microscopy in the 1970s, but little attention was paid to them for a long time. By a stroke of good luck, our screening identified these cells again and threw more light on their hidden role."
Yu Kitadate PhD, Division of Germ Cell Biology, National Institute for Basic Biology [NIBB], National Institutes of Natural Sciences, and Department of Basic Biology, School of Life Science, Graduate University for Advanced Studies (Sokendai), Higashiyama, Myodaiji, Okazaki, Japan.
Quantitative analyses of mice with increased or decreased FGF production revealed a simple mechanism controls migratory stem cells uptake of FGFs. Stem cells that consume more FGFs are more likely to make duplicate stem cells. Those that consume less are inclined to make differentiated cells. In this way, stem cells effectively compete with each other for the limited supply of FGFs in a stem cell niche. This leads the stem cell number to automatically adjust to the supply of FGFs.
This discovery of a new, and extremely simple, mechanism of stem cell control based on "competition for self-renewal promoting factors (aka: mitogens)" advances our understanding of the regulation of stem cells in tissues without a canonical, anatomically definable stem cell niche - a microenvironment sometimes called an "open niche".
"These findings may have important implications for the regulation of stem cell density in other tissue types."
Benjamin D. Simons PhD, The Wellcome Trust/Cancer Research, Gurdon Institute, University of Cambridge, Cambridge, United Kingdom.
The study: "Competition for Mitogens Regulates Spermatogenic Stem Cell Homeostasis in an Open Niche," is published in Cell Stem Cell.
Highlights
• Mouse spermatogenic stem cells (SSCs) migrate among their differentiating progeny
• Lymphatic endothelial cells near vasculature secrete FGFs that act as SSC mitogens
• SSCs tune their self-renewal and differentiation in response to FGF consumption
• Competition for limited supply of mitogen (FGFs) regulates SSC density homeostasis
Summary
In many tissues, homeostasis is maintained by physical contact between stem cells and an anatomically defined niche. However, how stem cell homeostasis is achieved in environments where cells are motile and dispersed among their progeny remains unknown. Using murine spermatogenesis as a model, we find that spermatogenic stem cell density is tightly regulated by the supply of fibroblast growth factors (FGFs) from lymphatic endothelial cells. We propose that stem cell homeostasis is achieved through competition for a limited supply of FGFs. We show that the quantitative dependence of stem cell density on FGF dosage, the biased localization of stem cells toward FGF sources, and stem cell dynamics during regeneration following injury can all be predicted and explained within the framework of a minimal theoretical model based on “mitogen competition.” We propose that this model provides a generic and robust mechanism to support stem cell homeostasis in open, or facultative, niche environments.
Authors
Yu Kitadate, David J. Jörg, Moe Tokue, Ayumi Maruyama, Rie Ichikawa, Soken Tsuchiya, Eri Segi-Nishida, Toshinori Nakagawa, Aya Uchida, Chiharu Kimura-Yoshida, Seiya Mizuno, Fumihiro Sugiyama, Takuya Azami, Masatsugu Ema, Chiyo Noda, Satoru Kobayashi, Isao Matsuo, Yoshiakira Kanai, Takashi Nagasawa, Yukihiko Sugimoto, Satoru Takahashi, *Benjamin D. Simons, and *Shosei Yoshida.
Acknowledgements
The authors thank Kyowa Hakko Kirin Co., Ltd. for anti-FGF8 antibody; Y. Mii for anti-HS antibodies; S. Gupta for support of ISH screening; E. Watanabe, T. Awasaki, and Yoshida laboratory members for discussions and encouragements; T. Ogawa for critical reading of the manuscript; and M. Noda for microarray analysis. We acknowledge support of the Functional Genomics Facility and the Spectrography and Bioimaging Facility, NIBB Core Research Facilities, and the Model Animal Research Facility, NIBB Bioresource Center for animal care. This work was supported in part by Grant-in-Aid for Scientific Research (KAKENHI from MEXT and JSPS; grant numbers JP25122719, JP24112526, and JP22770226 to Y. Kitadate; JP20116004, JP25114004, JP16H02507, and JP18H05551 to S.Y.), AMED-CREST (JP18gm1110005 to S.Y.), a Royal Society EP Abraham Research Professorship and a Wellcome Trust Senior Investigator Award (098357/Z/12/Z) to B.D.S., and AMED. B.D.S. and D.J.J. acknowledge core funding to the Gurdon Institute from the Wellcome Trust (092096) and CRUK (C6946/A14492).
Return to top of page
| |
|
Jan 8, 2019 Fetal Timeline Maternal Timeline News News Archive
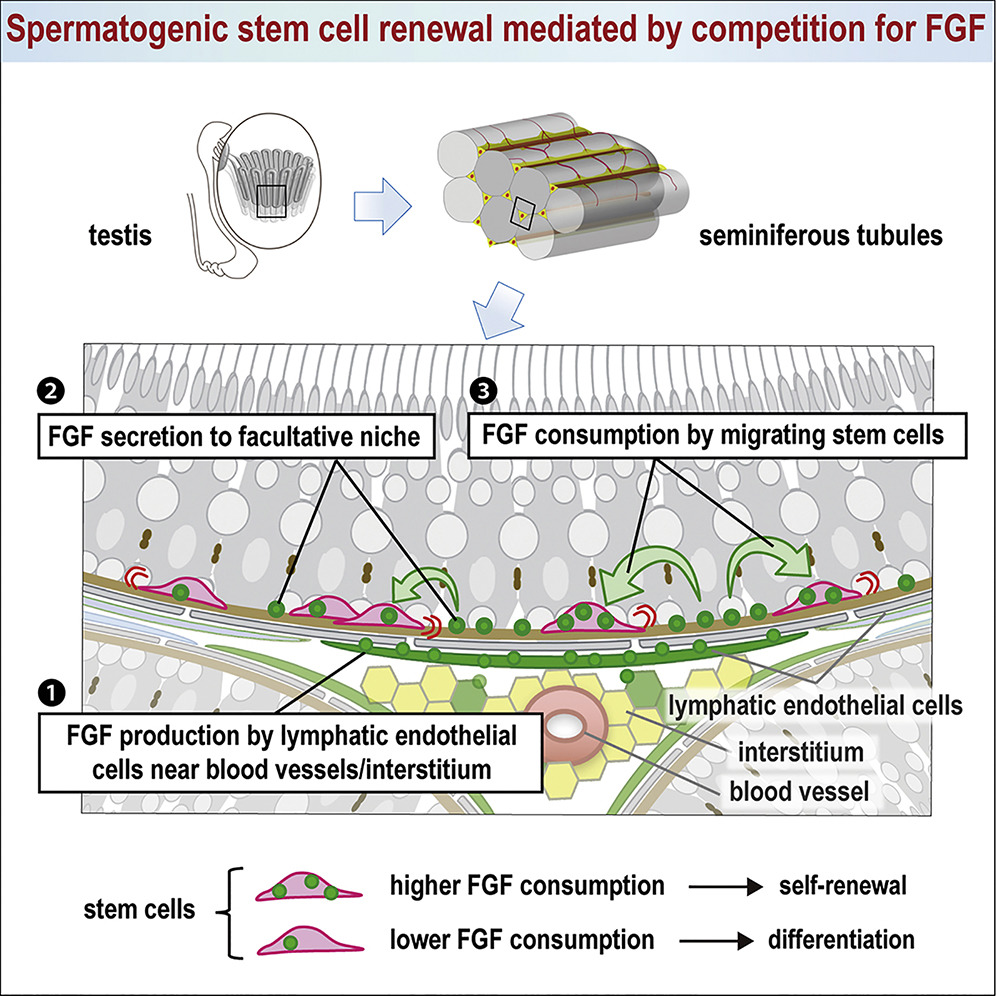 In the mouse testis, sperm stem cells (PINK) migrate over the basement membrane (BROWN), capture and consume FGFs (GREEN) produced by specialized Lymphatic Endothelial cells (LARGE GREEN CELLS). Competition between stem cells for limited FGFs, returns to homeostasis in a self-organized process. Image: NIBB
|



