|
|
Developmental Biology - Brain Structure
Proteins That Create Brain Boundaries
Two molecules regulate attraction and/or repulsion of cells in order to define distinct brain regions...
Boundaries between different regions of the brain are essential to its function. Research shows how molecular machinery located on the cell membrane is responsible for regulating formation of these boundaries. Specifically, how molecules Slit and Netrin regulate the attraction and/or repulsion of cells.
Makoto Sato and colleagues at Kanazawa University, Japan, report in iScience that these two diffusible molecules are essential for boundary formation in brains of the fly - Drosophila.
The visual center of the adult fly brain originates from two parts of the larval fly brain (1) the inner proliferation center (IPC) and (2) the outer proliferation center (OPC). Glial cells separate IPC neurons from OPC neurons (image) — ensuring each region gives rise to a distinct brain function.
Netrin becomes effective when attached to two receptor molecules known as Fra and Unc5. To examine the effects of Netrin, researchers inactivated it in fly larva visual centers. Altered flies now had IPC neurons penetrating their OPC neurons, which disrupted both OPC neurons and glial cells (image). These same effects were seen in flies with inactivated Fra and Unc5. Similarly, Slit becomes active only bound to its receptor Robo. Inactivation of either results in similar boundary defects.
These findings are important as guidance molecules are different from molecules that act on cell membranes. It was still very difficult to imagine how guidance molecules govern boundary formation, so Sato and his team created a mathematical model of functions by Slit and Netrin, to demonstrate that guidance molecules indeed regulate brain boundary formation.
The exchange of Slit and Netrin with their respective partners, between neurons and glial cells were simulated. Slit produced by glial cell always repels neurons. However, given that Netrin possesses attractive and repulsive properties, then how does Netrin function?
The research model shows that Netrin produced by neurons, attracts glial cells when its concentration is low – but repells glial cells at high concentration.
This balance between attraction and repulsion of neurons to glial cells regulates boundary formation between different brain regions, and links diffusible guidance molecules to boundary formation mechanisms in multicellular organisms.
"As these signaling pathways are evolutionarily conserved from insects to mammals, their role in establishing tissue borders may also be conserved across species", the scientists concluded. This explains how these pathways may prevent structural, and thereby functional, deformities in brains of higher species such as humans.
Inhibition of cell mixing also aids in keeping toxic cancerous cells, from invading healthy ones.
Summary
The brain consists of distinct domains defined by sharp borders. So far, the mechanisms of compartmentalization of developing tissues include cell adhesion, cell repulsion, and cortical tension. These mechanisms are tightly related to molecular machineries at the cell membrane. However, we and others demonstrated that Slit, a chemorepellent, is required to establish the borders in the fly brain. Here, we demonstrate that Netrin, a classic guidance molecule, is also involved in the compartmental subdivision in the fly brain. In Netrin mutants, many cells are intermingled with cells from the adjacent ganglia penetrating the ganglion borders, resulting in disorganized compartmental subdivisions. How do these guidance molecules regulate the compartmentalization? Our mathematical model demonstrates that a simple combination of known guidance properties of Slit and Netrin is sufficient to explain their roles in boundary formation. Our results suggest that Netrin indeed regulates boundary formation in combination with Slit in vivo.
Authors
Takumi Suzuki, Chuyan Liu, Satoru Kato, Kohei Nishimura, Hiroki Takechi, Tetsuo Yasugi, Rie Takayama, Satoko Hakeda-Suzuki, Takashi Suzukiand Makoto Sato.
Acknowledgements
The authors thank Masako Kaido for technical assistance, and are grateful to Benjamin Altenhein, Barry J. Dickson, James B. Skeath, Iris Salecker, S. Lawrence Zipursky, and Yuh-Nung Jan for antibodies and fly strains. They also thank Masaharu Nagayama and Hirofumi Notsu for expert advises on mathematical modeling and numerical simulations and Yohei Shinmyo for helpful comments on the manuscript. Thanks goes to Bloomington Stock Center, Vienna Drosophila RNAi Center, and DGRC, Kyoto, for fly strains and DSHB. This work was supported by CREST from JST (JPMJCR14D3 to M.S.), Grant-in-Aid for Scientific Research on Innovative Areas and Grant-in-Aid for Scientific Research (B) and (C) from MEXT (JP17H05739, JP17H05761, JP17H03542 to M.S., JP18H05099 to T.Y., JP26291047, JP16H06457 to Takashi Suzuki, and JP18K06250 to S.H.-S.), Sekisui Chemical Grant Program, Asahi Glass Foundation, and Takeda Science Foundation and Cooperative Research of 'Network Joint Research Center for Materials and Devices' (to M.S.).
Return to top of page
| |
|
Jan 14, 2019 Fetal Timeline Maternal Timeline News News Archive
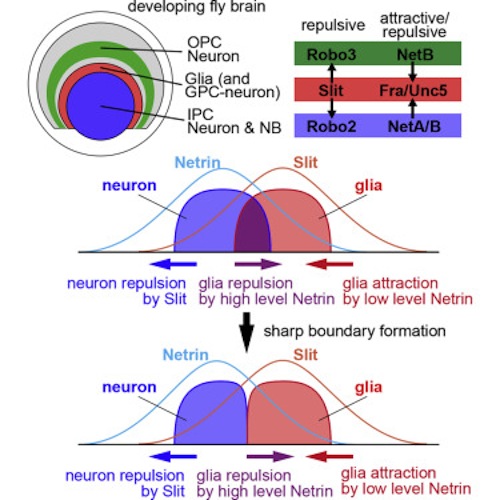 Different regions in the developing fly brain (CIRCLE) and how the 'guidance molecules' called Slit-Robo and Netrin inhibit cell mixing (GREEN, RED, PURPLE BARS). Credit: Kanazawa University.
|



