|
|
Developmental Biology - Aneuploidy
How Cells Fight Chromosome Imbalance
Biologists discover the immune system can eliminate cells with too many or too few chromosomes...
Most living cells have a defined number of chromosomes — each human cell has 23 pairs or 46 chromosomes, except for our germ (sperm and egg) cells. As cells divide, they can make errors that lead to a gain or loss in chromosomes, a situation usually very harmful to a cell.
Masschusetts Institute of Technology (MIT) biologists have identified how the immune system identifies a genetically imbalanced cell that needs to be eliminated. After gaining or losing chromosomes, cells send out signals to recruit immune cells called natural killer cells, to destroy abnormal cells.
This finding raises the possibility of harnessing the immune system to kill cancer cells, which nearly always have too many or too few chromosomes.
"If we can re-activate this immune recognition system, that would be a good way of getting rid of cancer cells."
Angelika Amon PhD, Koch Institute for Integrative Cancer Research, and the Kathleen and Curtis Marble Professor in Cancer Research, MIT Department of Biology, senior author of the study.
Stefano Santaguida PhD, research scientist, Koch Institute, is lead author of the paper, which appears in the June 19, 2018 issue of Developmental Cell.
"A downward spiral"
Before a cell divides, chromosomes replicate themselves and then line up in the middle of the cell. As the cell divides into two exact copies, half of these chromosomes are pulled into each cell, now known as daughter cells . If these chromosomes fail to separate properly, the process leads to an imbalanced number of chromosomes in each daughter cell — a state known as aneuploidy.
When aneuploidy occurs in embryonic cells, it is almost always fatal to the organism.
For human embryos, extra copies of any chromosome are lethal, with the exceptions of chromosome 21, which produces Down syndrome, or with chromosomes 13 and 18, which lead to developmental disorders known as Patau and Edwards syndromes. With the X and Y sex chromosomes, extra copies may cause various disorders but are not usually lethal.
In recent years, Amon's lab has been exploring an apparent paradox of aneuploidy:
• When normal adult cells become aneuploid, it impairs their ability to survive and proliferate
• However, cancer cells, which are nearly all aneuploid, can grow uncontrollably.
"Aneuploidy is highly detrimental in most cells. However, aneuploidy is highly associated with cancer, which is characterized by upregulated growth. So, a very important question is: If aneuploidy hampers cell proliferation, why are the vast majority of tumors aneuploid?"
Stefano Santaguida PhD, Department of Biology, Koch Institute for Integrative Cancer Research, Howard Hughes Medical Institute, Massachusetts Institute of Technology, Cambridge, MA, USA.
To try to answer that question, researchers wanted to find out more about how aneuploidy affects cells. Over the past few years, Santaguida and Amon studied what happens to cells immediately after they experience a mis-segregation of chromosomes, leading to imbalanced daughter cells. In this new study, they investigated the effects of this imbalance on the cell division cycle by interfering with the process of proper chromosome attachment to the spindle, the structure that holds chromosomes in place at the cell's equator before division. This interference leads some chromosomes to lag behind and get shuffled into the two daughter cells.
They found that after cells undergo their first division, in which some chromosomes were unevenly distributed, another cell division was initiated that produced even more chromosome imbalance, and significant DNA damage. Eventually, those cells stopped dividing altogether. "These cells are in a downward spiral where they start out with a little bit of a genomic mess, and it just gets worse and worse," Amon adds.
Targeting aneuploidy
As genetic errors accumulate, aneuploid cells eventually become too unstable to keep dividing. In this senescent state, they start producing inflammation-inducing molecules such as cytokines. When the researchers exposed these cells to immune cells called natural killer cells most of the aneuploid cells were destroyed.
"For the first time, we are witnessing a mechanism that might provide a clearance of cells with imbalanced chromosome numbers."
Stefano Santaguida PhD
In future studies, researchers hope to more precisely determine how aneuploid cells attract natural killer cells, and whether other immune cells are involved. They also need to understand how tumor cells are able to evade immune clearance, and if it is possible to restart immune clearance in patients with cancer.
About 90 percent of solid tumors and 75 percent of blood cancers are aneuploid.
Amon: "At some point, cancer cells, which are highly aneuploid, are able to evade this immune surveillance. We have really no understanding of how that works. If we can figure this out, that probably has tremendous therapeutic implications, given the fact that virtually all cancers are aneuploid."
Highlights
• p53 activation is a potential, but not obligatory, outcome of chromosome mis-segregation
• Chromosome segregation errors lead to replication stress and DNA damage
• Aneuploidy drives genome instability and evolution of complex karyotypes
• Aneuploid cells with complex karyotypes are cleared by natural killer cells
Summary
Aneuploidy, a state of karyotype imbalance, is a hallmark of cancer. Changes in chromosome copy number have been proposed to drive disease by modulating the dosage of cancer driver genes and by promoting cancer genome evolution. Given the potential of cells with abnormal karyotypes to become cancerous, do pathways that limit the prevalence of such cells exist? By investigating the immediate consequences of aneuploidy on cell physiology, we identified mechanisms that eliminate aneuploid cells. We find that chromosome mis-segregation leads to further genomic instability that ultimately causes cell-cycle arrest. We further show that cells with complex karyotypes exhibit features of senescence and produce pro-inflammatory signals that promote their clearance by the immune system. We propose that cells with abnormal karyotypes generate a signal for their own elimination that may serve as a means for cancer cell immunosurveillance.
Authors
Stefano Santaguida, Amelia Richardson, Divya Ramalingam Iyer, Ons M'Saad, Lauren Zasadil, Kristin A. Knouse, Yao Liang Wong, Nicholas Rhind, Arshad Desai and Angelika Amon.
Acknowledgements
The research was funded, in part, by the National Institutes of Health, the Kathy and Curt Marble Cancer Research Fund, the American Italian Cancer Foundation, a Fellowship in Cancer Research from Marie Curie Actions, the Italian Association for Cancer Research, and a Koch Institute Quinquennial Cancer Research Fellowship.
Return to top of page
| |
|
Jan 21, 2019 Fetal Timeline Maternal Timeline News News Archive
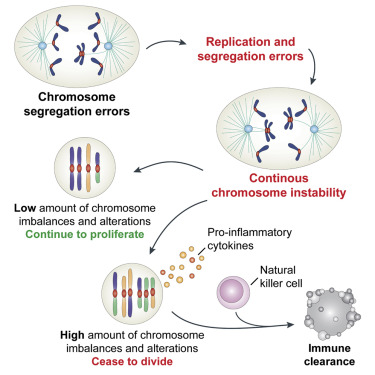 Cells with abnormal karyotypes generate a signal for their own elimination. This may be a means for immune system surveillance for cancer cell. Credit: M.I.T.
|



