|
|
Developmental Biology - Body Plan
The Long and the Short of It
Scientists accidentally engineer mice with unusually short and long tails...
Researchers from two groups studying mouse development have accidentally created mice with unusually long and unusually short tails. Their findings, publishing January 17 in the journal Developmental Cell, offer new insights into some key aspects controlling the length of mouse tails in mice and have implications for understanding what happens when developmental pathways go awry.
"The same regulatory networks that control how a body pattern is formed, are often coopted for other developmental processes," explainss Moisés Mallo, a researcher at Instituto Gulbenkian de Ciência in Lisbon, Portugal, and senior author of one of the two papers. "Studying these networks can give us relevant information even for understanding pathological processes," such as causes of diseases.
Both groups' findings are related to a gene called Lin28, which was already known to have a role in regulating body size and metabolism, among other functions.
"We were trying to make mouse model of Lin28-driven cancer, and were surprised to find these mice had super long tails with more vertebrae," says George Daley PhD, investigator and dean at Harvard Medical School and senior author of the paper. His team was studying the Lin28/let-7 pathway, which regulates developmental timing and has been implicated in several types of cancer.
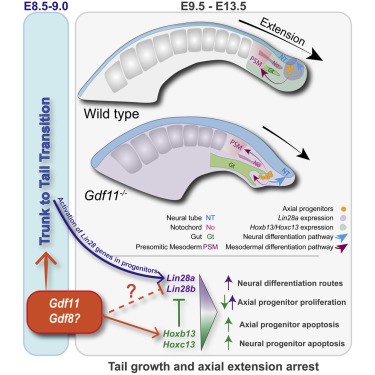
Mallo, on the other hand, was studying a gene called Gdf11, which was already known to be involved in triggering the development of the tail during embryonic development. In his lab, they found that mice with Gfd11 mutations had tails that were shorter and thicker than those of regular mice. "They also contained a fully grown neural tube inside, as opposed to a normal tail that is essentially made of vertebrae," Mallo says. "We were able to pinpoint the Lin28 and Hox13 genes as key regulators of tail development downstream from Gdf11."
Both pathways relate to the development of somites, which give rise to important structures in the vertebrate body plan. These blocks of cells eventually differentiate into dermis, skeletal muscle, cartilage, tendons, and vertebrae. As mammals develop, the somites are laid down sequentially along the body axis. Lin28 plays a role in regulating the timing of this repetitive process.
"From my perspective, one of the most important findings of our work is that a group of multipotent cells that build both the somites and the spinal cord are regulated by fundamentally different genetic networks and have different cell competences at two consecutive stages of development," Mallo says. "This finding goes beyond the trunk to tail transition, possibly acquiring relevance in pathological processes like the initiation of metastasis."
"There are also important implications in this research for understanding evolution," says Daisy Robinton, a researcher at Harvard and first author of the study from Daley's lab. "Anterior-posterior axis elongation is an important feature in bilateral animals, and natural selection has created a variety of tail lengths to suit different evolutionary pressures. Until now, little was known about how length is controlled and how the manipulation of genetics can impact morphogenesis."
Robinton says the next steps for the Daley lab are to address the question of whether Lin28/let-7 acts similarly in other organ systems, as well as to explore more deeply how this pathway influences cell fate decisions during mammalian development.
For Mallo, future work will focus on uncovering further molecular details of how these players modulate the activity of tail bud progenitors and deepening the understanding of how these molecular interactions are mediated.
Highlights
• Gdf11 mutant tail buds have an enlarged progenitor pool biased toward neural fates
• Different gene networks regulate axial progenitor activity at trunk and tail levels
• Tail bud progenitors do not rely on Oct4 for axial extension
• Lin28 genes promote and Hox13 genes restrict tail bud progenitor expansion
Summary
During the trunk-to-tail transition, axial progenitors relocate from the epiblast to the tail bud. Here, we show that this process entails a major regulatory switch, bringing tail bud progenitors under Gdf11 signaling control. Gdf11 mutant embryos have an increased number of such progenitors that favor neural differentiation routes, resulting in a dramatic expansion of the neural tube. Moreover, inhibition of Gdf11 signaling recovers the proliferation ability of these progenitors when cultured in vitro. Tail bud progenitor growth is independent of Oct4, relying instead on Lin28 activity. Gdf11 signaling eventually activates Hox genes of paralog group 13, which halt expansion of these progenitors, at least in part, by down-regulating Lin28 genes. Our results uncover a genetic network involving Gdf11, Lin28, and Hox13 genes controlling axial progenitor activity in the tail bud.
Authors
Rita Aires, Luisade Lemos, Ana Nóvoa, Arnon Dias Jurberg, Bénédicte Mascrez, Denis Duboule and Moisés Mallo.
Return to top of page
| |
|
Jan 24, 2019 Fetal Timeline Maternal Timeline News News Archive
 Trying to make mice reflecting the affects of the cancer gene Lin28, researchers stumbled across the mechanism for vertebrate growth in their tails. In mice with the Gf11 gene mutation whose tails are typically short and thick, the vertebrae in the tail also contained fully grown neural tubes. This feature is not seen in typical mice tail vertebrae. Image: Harvard University.
|



