|
|
Developmental Biology - Gastrulation
Gastrulation In Artificial Embryos
Scientists generate key life event in artificial mouse 'embryo' created from stem cells...
The creation of artificial embryos has moved a step forward after an international team of researchers used mouse stem cells to produce artificial embryo-like structures capable of gastrulation, a key step in the life of any embryo.
The team, led by Professor Magdalena Zernicka-Goetz at the University of Cambridge, previously created a much simpler structure resembling a mouse embryo in culture, using two types of stem cells - the body's 'master cells' - and a 3D scaffold on which they can grow. Now, in the journal Nature Cell Biology, Professor Zernicka-Goetz and colleagues reveal how they developed embryo-like structures using not just two, but three types of stem cells and reconstructed gastrulation.
Once a mammalian egg has been fertilised, it divides multiple times generating a free-floating ball made up of three types of stem cells. At the 'blastocyst' stage, stem cells that will eventually make all the cells of the future body - embryonic stem cells (ESCs) - cluster together towards one end of the embryo. Another stem cell type — the extra-embryonic trophoblast stem cells (TSCs) — will form the placenta. The third type of stem cells are called primitive endoderm stem cells (PESCs) and form the yolk sac, providing essential nutrients.
In March 2017, Professor Zernicka-Goetz and colleagues used a combination of genetically-modified mouse ESCs and TSCs, within a 3D 'jelly' scaffold called an extracellular matrix, to grow a structure capable of self assembly into the architecture very closely resembling a natural embryo. A remarkable degree of communication between the two stem cell types led them to place themselves into an embryo. However, gastrulation — described by the eminent biologist Lewis Wolpert as "truly the most important time in your life" — was missing.
Gastrulation is the point at which the embryo transforms from being a single layer to three layers: an inner layer (endoderm), middle layer (mesoderm) and outer layer (ectoderm), determining which tissues or organs the cells will then develop into. By adding PESCs, the 'embryo' underwent gastrulation, organising itself into three body layers as seen in all animals, reflecting the timing, architecture and patterns of gene activity found in a natural embryo.
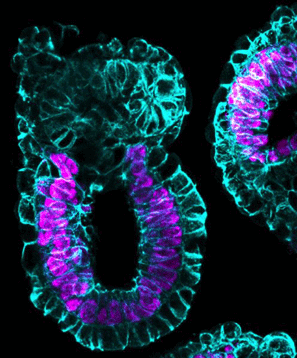 Gastrulation in artificial embryo.
Gastrulation in artificial embryo.
Zernicka-Goetz lab, University of Cambridge, UK.
"Proper gastrulation in normal development is only possible if you have all three types of stem cells. In order to reconstruct this complex dance, we had to add the missing third stem cell," says Professor Zernicka-Goetz. "By replacing the jelly that we used in earlier experiments with this third type of stem cell, we were able to generate structures whose development was astonishingly successful."
By applying these studies side-by-side, it should be possible to learn a great deal about the fundamental aspects of the first stages of mammalian development. In fact, such comparisons should enable scientists to study events that happen beyond day 14 in human pregnancies, but without using 14-day-old human embryos. UK law permits embryos to be studied in the laboratory only up to this period.
Abstract
Embryonic stem cells can be incorporated into the developing embryo and its germ line, but, when cultured alone, their ability to generate embryonic structures is restricted. They can interact with trophoblast stem cells to generate structures that break symmetry and specify mesoderm, but their development is limited as the epithelial–mesenchymal transition of gastrulation cannot occur. Here, we describe a system that allows assembly of mouse embryonic, trophoblast and extra-embryonic endoderm stem cells into structures that acquire the embryo’s architecture with all distinct embryonic and extra-embryonic compartments. Strikingly, such embryo-like structures develop to undertake the epithelial–mesenchymal transition, leading to mesoderm and then definitive endoderm specification. Spatial transcriptomic analyses demonstrate that these morphological transformations are underpinned by gene expression patterns characteristic of gastrulating embryos. This demonstrates the remarkable ability of three stem cell types to self-assemble in vitro into gastrulating embryo-like structures undertaking spatio-temporal events of the gastrulating mammalian embryo.
Authors
Berna Sozen, Gianluca Amadei, Andy Cox, Ran Wang, Ellen Na, Sylwia Czukiewska, Lia Chappell, Thierry Voet, Geert Michel, Naihe Jing, David M. Glover and Magdalena Zernicka-Goetz.
Acknowledgements
The research was funded by the European Research Council and Wellcome.
The authors thank colleagues in the M.Z.G. laboratory for insightful comments. The M.Z.G. laboratory is supported by grants from the European Research Council (669198) and the Wellcome Trust (098287/Z/12/Z). B.S. is also supported by Akdeniz University, Turkey. T.V. and L.C. are funded by Wellcome. T.V. is also funded by the University of Leuven, Belgium (PFV/10/016). The authors thank A. Weberling, M. Mole, N. Christodoulou, C. Kyprianou and J. Guo for their help, A. Hupalowska for inspiration for a model in Fig. 7f, and L. Wittler, I. Urban, A. Landsberger, C. Schick and H. Schlenger for technical support.
Return to top of page
| |
|
Feb 12, 2019 Fetal Timeline Maternal Timeline News
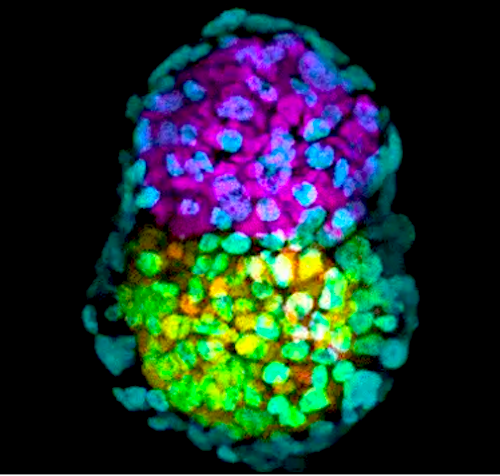 Synthetic embryo-like structure made of three stem cells types in yellow, pink and green. Image credit: Zernicka-Goetz lab, University of Cambridge, UK.
|




