|
|
Developmental Biology - Embyro Implantation
Disrupting Embryo Implantation
Folliculin gene defects are implicated in infertility, some forms of cancer, and in cell growth disorders...
New information is unfolding about genetic influence in early pregnancy. As the tiny, continually dividing cell mass, known as the blastocyst, travels through the oviduct and lodges into the wall of the uterus, it must exit a pre-implantation state and be ready to enter post-implantation development.
Failure of embryos to embed in uterine lining is the main cause of human infertility. Many pregnancies suddenly stop at this stage, often before a woman realizes she has conceived. Important changes inside blastocyst cells are critical in guiding a pregnancy successfully through implantation.
"We know little about the genes that control these cells during implantation," explains Hannele Ruohola-Baker, professor of biochemistry at the University of Washington School of Medicine and associate director of the UW Medicine Institute for Stem Cell and Regenerative Medicine.
She and Julie Mathieu, assistant professor of comparative medicine at the UW School of Medicine, have designed a CRISPR-Cas9 gene editing screen to study the genes involved in pre- and post-implantation embryos. They collaborated with Patrick Paddison, a Fred Hutchinson Cancer Research Center scientist who studies functional genomics. Their investigation started with a whole genome CRISPR screen for relevant genes.
Many genes appear important in exiting pre-implantation. But, folliculin (FLCN), was a gene suspected of being a tumor suppressor.

Embryo implanted in uterine lining (RED).
"We found that when the FLCN gene is missing, cells in the blastocyst maintain their pluripotent state and can't advance into their implantation stage," Ruohola-Baker explains. These findings are published in the Feb. 7 Nature Communications.
"By using CRISPR-Cas9 editing to knock out this gene, we saw that it wasn't needed for pre-implantation embryo development, but embryonic stem cells couldn't move forward into the post-implantation state if it was removed," they observed.
Researchers found by that by inhibiting the Wnt pathway, they could rescue blastocyst cells. Wnt is a cell signal regulating early development, which later maintains structure in body tissues. Wnt also tells cells to self-renew.
Experiments indicate when the FLCN gene is knocked out by CRISPR editing, Wnt stymies blastocyst continuance.
Patients with naturally occurring mutations in the FLCN gene, such as those with Birt Hogg Dube syndrome, had problems with a transcription factor called TFE3. Transcription factors dictate which genes are turned on to make proteins, and where and when gene production takes place.
In cells with FLCN mutations, the TFE3 transcription factor mistakenly stays inside the nucleus (a cell's management center). At that point in the embryonic timetable, it should be in the cell cytoplasm, the contents outside of the nucleus.
The presence of FLCN mutation in the nucleus of stem cells, appears to inhibit embryonic development. Researchers hypothesize activation of Wnt by TFE3 cells missing the FLCN gene, prevents cells leaving a preimplantation state.
Researchers went on to look for proteins that bind to folliculin. In collaboration with developmental biologist Randall Moon, professor of pharmacology at the UW School of Medicine, they found protein complexes containing folliculin differ in the pre- and post-implantation state. Finding components of these proteins is a big clue to mechanisms of implantation transition - as they are associated with the mTor (mammalian target of rapamycin) pathway.
Part of the survival monitoring system in cells, mTor helps cells sense a variety of environmental cues to manage their energy and nutrients. The mTor pathway is also under investigation in a number of diseases, including type 2 diabetes, aging disorders, and cancer.
Learning the genetic controls, protein interactions and signaling pathways in embryonic development and infertility could help identify mechanisms of diseases common in later life. Detecting which pathways should be targeted to reverse symptoms in certain kidney cancer and other forms of cancer, even in lung diseases, would benefit us all.
Abstract
To reveal how cells exit human pluripotency, we designed a CRISPR-Cas9 screen exploiting the metabolic and epigenetic differences between naïve and primed pluripotent cells. We identify the tumor suppressor, Folliculin(FLCN) as a critical gene required for the exit from human pluripotency. Here we show that FLCN Knock-out (KO) hESCs maintain the naïve pluripotent state but cannot exit the state since the critical transcription factor TFE3 remains active in the nucleus. TFE3 targets up-regulated in FLCN KO exit assay are members of Wnt pathway and ESRRB. Treatment of FLCN KO hESC with a Wnt inhibitor, but not ESRRB/FLCN double mutant, rescues the cells, allowing the exit from the naïve state. Using co-immunoprecipitation and mass spectrometry analysis we identify unique FLCN binding partners. The interactions of FLCN with components of the mTOR pathway (mTORC1 and mTORC2) reveal a mechanism of FLCN function during exit from naïve pluripotency.
Authors
J. Mathieu, D. Detraux, D. Kuppers, Y. Wang, C. Cavanaugh, S. Sidhu, S. Levy, A. M. Robitaille, A. Ferreccio, T. Bottorff, A. McAlister, L. Somasundaram, F. Artoni, S. Battle, R. D. Hawkins, R. T. Moon, C. B. Ware, P. J. Paddison & H. Ruohola-Baker.
Acknowledgements
The stem cell lines for this study were developed by Carol Ware, professor of comparative medicine at the UW School of Medicine, and director of the Tom and Sue Ellison Stem Cell Core Facility. Yulian Wang, research assistant professor of computer science and engineering at the UW Paul G. Allen School, led the project's computational biology aspects. The Feb. 7 Nature Communications paper, "Folliculin regulates mTORC1/2 and WNT pathways in early human pluripotency," provides a full list of collaborators for this project.
The authors thank members of the Ruohola-Baker laboratory for helpful discussions throughout this work. They thank Jennifer Hesson, Ammar Alghadeer, and Asis Hussein for technical help, and Dr. Rudolf Jaenisch for providing WIBR3 5iLA cells. The small-molecule screen was done in Quellos High Throughput Screening Core, Institute for Stem Cell and Regenerative Medicine, University of Washington School of Medicine. This work is supported in part by the University of Washington’s Proteomics Resource (UWPR95794). R.T.M. is an Investigator, and A.M.R. is Associate, of the HHMI. This work is supported by the ISCRM Innovation Pilot Award for J.M. and grants from the National Institutes of Health R01GM097372, R01GM97372-03S1, and R01GM083867 for H.R.-B., 1P01GM081619 for C.B.W. and H.R.-B., and the NHLBI Progenitor Cell Biology Consortium (U01HL099997; UO1HL099993) for P.J.P., C.B.W. and H.R.-B.
Return to top of page
| |
|
Feb 13, 2019 Fetal Timeline Maternal Timeline News
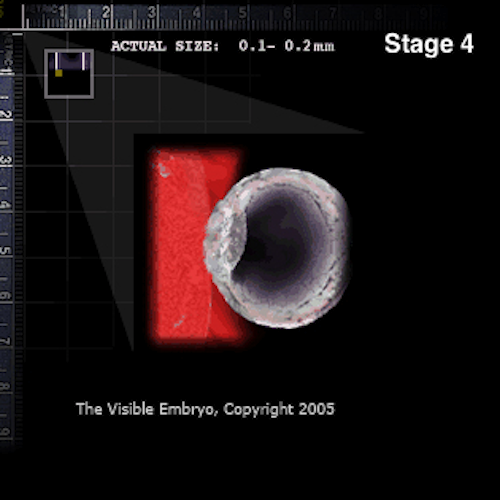 As the blastocyst enters the uterus free from the zona, the outer layer of trophoblast cells secrete an enzyme to erode the epithelial lining of the uterus and allow the blastocyst to implant.. Credit: The Visible Embryo.
|




