|
|
Developmental Biology - Telomeres
What Controls Telomeres On Our Chromosomes?
Telomere degeneration can lead to total erosion of genetic material triggering cancer and diseases of ageing...
The tips of our chromosomes are covered by structures called telomeres. Often they are compared to the plastic cover on the tips of shoelaces. When telomeres do not work properly, it can lead to the total erosion of our genetic material, possibly triggering cancer and age related diseases.
Telomeres work like a protective cap to prevent our genetic material from unfolding and corroding away.
In a study now published in the EMBO Journal, a research team from Instituto Gulbenkian de Ciencia (IGC), and led by Jose Escandell and Miguel Godinho Ferreira, have discovered a key aspect regulating telomeres.
An increasing number of human syndromes are attributed to telomere malfunction. Recently identified is a protein complex malfunction in CST — which is responsible for maintaining telomeres. Deficiencies in CST give rise to a telomeropathy known as Coats Plus, a syndrome genetically inherited and characterized by abnormalities in the gastrointestinal system, bones, brain, eyes and more.
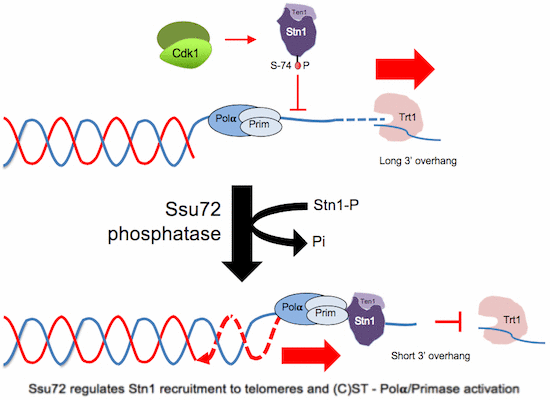
IGC researchers have now unveiled how regulation of a component - S - in the CST complex works. They discovered that STN1 (the protein that corresponds to S) is regulated by insertion of phosphorus into the protein — which can also be reversed by another enzyme, phosphatase SSU72.
S allows telomere duplication and regulation of telomerase to elongate telomeres. Research also shows the process is identical both in yeast and in humans — meaning regulation of S is conserved through evolution of species, revealing its importance to the process of correct cell function.
This opens possiblities for discovery of therapies for diseases associated with telomere defects in S.
"The unanticipated role of this evolutionary conserved phosphatase is reminiscent of regulation of the cell cycle by phosphatases that counteract the role of kinases, thus re-establishing the rule 'once and only once' in cell cycle processes."
Miguel Godinho Ferreira PhD, Instituto Gulbenkian de Ciência, Oeiras, Portugal and Institute for Research on Cancer and Aging (IRCAN), INSERM, Nice, France.
"With this work, we now better understand how telomere regulation works, key to cancer - ageing."
Jose Miguel Escandell PhD, Instituto Gulbenkian de Ciência, Oeiras, Portugal and first author on the paper.
Abstract
Telomeres, the protective ends of eukaryotic chromosomes, are replicated through concerted actions of conventional DNA polymerases and elongated by telomerase, but the regulation of this process is not fully understood. Telomere replication requires (Ctc1/Cdc13)-Stn1-Ten1, a telomeric ssDNA-binding complex homologous to RPA. Here, we show that the evolutionarily conserved phosphatase Ssu72 is responsible for terminating the cycle of telomere replication in fission yeast. Ssu72 controls the recruitment of Stn1 to telomeres by regulating Stn1 phosphorylation at Ser74, a residue located within its conserved OB-fold domain. Consequently, ssu72? mutants are defective in telomere replication and exhibit long 3?-ssDNA overhangs, indicative of defective lagging-strand DNA synthesis. We also show that hSSU72 regulates telomerase activation in human cells by controlling recruitment of hSTN1 to telomeres. These results reveal a previously unknown yet conserved role for the phosphatase SSU72, whereby this enzyme controls telomere homeostasis by activating lagging-strand DNA synthesis, thus terminating the cycle of telomere replication.
Authors
Jose Miguel Escandell, Edison SM Carvalho, Maria Gallo-Fernandez, Clara C Reis, Samah Matmati, Inês Matias Luís, Isabel A Abreu, Stéphane Coulon and Miguel Godinho Ferreira.
The authors declare no conflict of interest.
Acknowledgements
The authors thank A Bianchi, AM Carr, JP Cooper, T Nakamura, P Nurse, and M Sato for sharing their strains and J Lingner and T Wang for sharing plasmid constructs. We are grateful to K Tomita, L Jansen, R Carlos, and A Sridhar for reading the manuscript and to MGF laboratory for critical comments and discussions. This work was supported by the Portuguese Fundação Ciência e tecnologia (FCT) project number PTDC/BEX-BCM/5179/14. JME is supported by PTDC/BEX-BCM/5179/14 and PIEF-GA-2013-624759, and ESMC is supported by SFRH/BD/113754/2015. SM is supported by the Ligue Nationale Contre le Cancer (LNCC, Equipe Labellisée Vincent Géli). SC is supported by Projet Fondation ARC and by the Agence Nationale de la Recherche (ANR-16-CE12 TeloMito). IML acknowledges FCT for PhD fellowship funding (PD/BD/113982/2015) under the MolBioS PhD-Program (PD/00133/2012). IAA acknowledges IF/00764/2014 Research unit GREEN-IT “Bioresources for Sustainability” (UID/Multi/04551/2013). Mass spectrometry analyses were performed at UniMS (ITQB/iBET). MGF was supported by the HHMI International Early Career Scientist program.
Funding
Portuguese Fundação Ciência e tecnologia (FCT) PTDC/BEX-BCM/5179/14SFRH/BD/113754/2015PD/BD/113982/2015IF/00764/2014UID/Multi/04551/2013
EC | FP7 | FP7 People: Marie-Curie Actions PEOPLE PIEF-GA-2013-624759
Ligue Contre le Cancer (Equipe Labellisée Vincent Géli)
Agence Nationale de la Recherche (ANR) ANR-16-CE12 TeloMito
MolBioS PhD-Program PD/00133/2012
HHMI International Early Career Scientist Program
Return to top of page
| |
|
Mar 1 2019 Fetal Timeline Maternal Timeline News
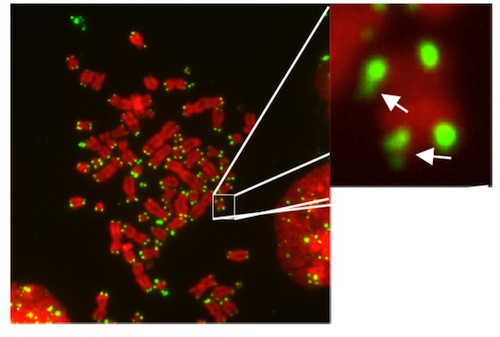 Telomeres (GREEN) at the tips of chromosomes (RED). Image: Jose Escandell, IGC.
|