|
|
Developmental Biology - Heart
Immune Cells Help Form Heart Valves
New info on the critical role of macrophage cells in keeping heart valves flexible...
UCLA researchers for the first time have identified the origin of an immune cell that plays a critical role in the formation of healthy heart valves. These findings could pave the way for new treatments for heart valve disorders, which can be caused by congenital defects, aging or disease.
Their study, led by Dr. Atsushi "Austin" Nakano, a UCLA associate professor of molecular, cell and developmental biology and member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA, was published in the journal Developmental Cell.
Building on previous research by Nakano, which showed the embryonic heart tube produces blood progenitor (originator) cells, the new study finds these cells generate specialized immune cells called macrophages. Researchers also revealed these heart-derived macrophage cells consume excess tissue, a skill making them indispensable to forming and maintaining heart valves.
The human heart has four valves - tissue-paper thin membranes that constantly open and close to control blood flow through the heart. When valves don't function properly, blood flow to the body is disrupted, which strains the heart and can lead to heart failure, stroke or sudden death.
"When valves are seriously damaged, they cannot be fixed; replacement surgery is the only option," Nakano said. "Identifying cells that contribute to valve health could reveal targets for new, less-invasive therapies."
Currently, doctors have two options for replacement valves: mechanical valves, which require lifelong use of blood-thinning medications; and biological valves, which are made from cow, pig or human heart tissue, and which usually need to be replaced every 10 to 15 years.
Because replacement valves often require replacements of their own - notably among children as they outgrow their valve replacements multiple times before they reach adulthood - and because of the risks associated with any surgery that alters the heart, Nakano says new methods for treating valve disorders are urgently needed.
In a 2013 study using mice, Nakano and colleagues in his lab discovered that the heart tube - the embryonic 'heart' before it begins pumping blood — contributes to the production of the body's early blood cells, called blood progenitor cells. Just like stem cells can form any type of cell in the body, blood progenitor cells can create several different types of blood and immune cells. But unlike stem cells, blood progenitor cells can not self-renewing throughout an organism's entire lifespan.
"Ever since we discovered that the heart tube produces some blood progenitor cells, we have been trying to figure out why," Nakano said. "Blood progenitor cells are generated in much greater numbers in other parts of the developing embryo. Having the heart tube produce blood progenitor cells is like having a small, not-very-productive factory just down the street from a larger, more productive factory. If both factories produce the same thing - in this case blood progenitor cells - why not just have one big factory?"
Answering that question was not a simple task, in part because the heart tube changes shape and begins beating within just days of its development. And with each heartbeat, blood and immune cells from all over the developing embryo flow into the heart and back out again, making it difficult to determine the origin of blood cells in the heart.
In the new study, which also used mice, the team eliminated the other blood and immune cells from the equation by removing a heart tube before it began pumping blood and continuing its growth in a lab dish. With no circulating blood to contaminate their sample, the team observed that the heart-derived blood progenitor cells were producing macrophages.
Macrophages (meaning "big eaters" in Greek) are a type of white blood cell and part of the immune system. They engulf and digest cell debris, as they travel through our blood. Previous research shows they exist in heart valves, but Nakano's team is the first to discover that by eating up excess cellular debris, they keep heart valves paper-thin and hyper-efficient. This begins in the developing embryo as the heart is forming and continues throughout our lifespan.
"Macrophages were known to exist in heart valves, but nobody had nailed down when they arrived there and where they came from until we watched them develop in the heart tube," Nakano explains.
To test just how essential heart-derived macrophages are to valve formation and remodeling, their production was blocked to see if it had any effect. Scientists found that other macrophages in the body - those in circulating blood - traveled to the heart, but weren't very effective at remodeling heart valves. Without heart-tube derived macrophages, the heart valves remained thick and unwieldy.
"This showed us that the macrophages generated in the heart tube are particularly adept at eating up excess tissue," Nakano said. "This makes them essential not just to heart valve formation, but to heart valve maintenance throughout life."
Nakano hopes that this discovery will pave the way to solving human heart valve conditions, perhaps by boosting or inhibiting heart-derived macrophages' activity to regulate heart valve formation. As those macrophages remain in the body throughout people's lives, it could one day be possible to target them to treat valve problems that develop later in life.
Highlights
• Hemogenic endocardium is a source of cardiac macrophages in cardiac valves
• Endocardial macrophages are more phagocytic than macrophages from other source(s)
• Genetic ablation of endocardial macrophages induces defects in valve formation
• Endocardially derived macrophages are indispensable for normal valve remodeling
Summary
During mammalian embryogenesis, de novo hematopoiesis occurs transiently in multiple anatomical sites including the yolk sac, dorsal aorta, and heart tube. A long-unanswered question is whether these local transient hematopoietic mechanisms are essential for embryonic growth. Here, we show that endocardial hematopoiesis is critical for cardiac valve remodeling as a source of tissue macrophages. Colony formation assay from explanted heart tubes and genetic lineage tracing with the endocardial specific Nfatc1-Cre mouse revealed that hemogenic endocardium is a de novo source of tissue macrophages in the endocardial cushion, the primordium of the cardiac valves. Surface marker characterization, gene expression profiling, and ex vivo phagocytosis assay revealed that the endocardially derived cardiac tissue macrophages play a phagocytic and antigen presenting role. Indeed, genetic ablation of endocardially derived macrophages caused severe valve malformation. Together, these data suggest that transient hemogenic activity in the endocardium is indispensable for the valvular tissue remodeling in the heart.
Authors
Ayako Shigeta, Vincent Huang, Jonathan Zuo, Rana Besada, Yasuhiro Nakashima, Yan Lu, Yichen Ding, Matteo Pellegrini, Rajan P. Kulkarni, Tzung Hsiai, Arjun Deb, Bin Zhou, Haruko Nakano and Atsushi Nakano.
The authors declare no conflict of interest.
Acknowledgements
The research was supported by the National Institutes of Health. Nakano is on the advisory board of the Japan-based startup company Myoridge, which is developing technology to produce cardiac cells from induced pluripotent stem cells.
The research was made possible through tissue samples provided by colleagues in Houston, Texas, Copenhagen and Sydney.
The work was supported by grants from the National Institutes of Health's National Institute of General Medical Sciences under grants R01 GM051923-20 and F32 GM128322, the Cure Alzheimer's Fund, the Muscular Dystrophy Association (MDA-419143), Genentech and by Project ALS.
The authors thank Drs. Alvaro Sagasti, Karen Lyons, and Hanna K.A. Mikkola (UCLA) for the critical reading of the manuscript and Dr. Hajime Yokota for the assistance in bioinformatics analyses. Dr. Kouki Morizono (UCLA) advised on the macrophage phagocytosis assay. Technical support was provided by the BSCRC flow cytometry core, the BSCRC sequencing core, and the UCLA histology core. This work was supported by NIH, United States (HL127427 to A.N., HL129178 and HL137241 to A.D., and HL111437 and HL129727 to T.H.). The authors were supported by fellowships from the Japanese Respiratory Foundation-Pfizer (A.S.), the Howard Hughes Medical Institute (V.H.), the UCLA Program for Excellence in Education and Research in the Sciences (PEERS) (R.B.), the Japanese Circulation Society (Y.N.), and the Uehara Memorial Foundation (Y.N.).
Return to top of page
| |
|
Mar 6 2019 Fetal Timeline Maternal Timeline News
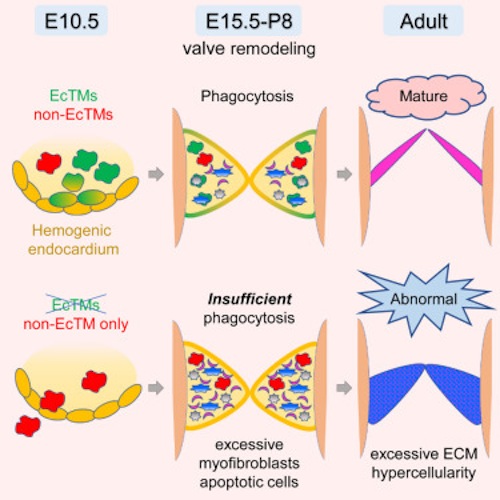 Experiments on mice reveal hemogenic endocardium, the innermost layer of tissue lining the chambers of the primitive heart, is the best source of macrophage cells which ingest (phagocytosis) foreign and infectious microorganisms and keep heart valves trim and flexible throughout our lives. Image: Developmental Biology UCLA.
|




