|
|
Developmental Biology - Infant Immunization
Mapping A Baby's First Week
For the first time, newborn's first seven days are molecularly mapped...
Co-led by the MRC Unit The Gambia at the London School of Hygiene & Tropical Medicine, a new study lifts the lid on what genes are turned on, what proteins are being made and what metabolites are changing in the first seven days of human life. The research mapping this developmental pathway in a newborn's life, published in Nature Communications, could transform our understanding of health and disease in babies.
Newborn babies are the most vulnerable population when it comes to infectious disease. Establishing key pathways in early development could help measure the impact of factors such as diet, disease and maternal health, as well as key interventions like vaccines.
The study was conducted by the Expanded Program on Immunization Consortium (EPIC) research team, which includes MRC Unit The Gambia; London School of Hygiene & Tropical Medicine; Boston Children's Hospital; the University of British Columbia; and, the Papua New Guinea Institute of Medical Research.
The first week of a newborn's life is a time of rapid biological change as the baby adapts to living outside the womb, suddenly exposed to new bacteria and viruses, yet surprisingly little is known about these early changes. One of the biggest challenges in gathering data on newborn development has been sourcing a large enough blood sample for comprehensive profiling from a tiny newborn. The team overcame this with pioneering laboratory techniques applied on less than half a teaspoon of blood.
By using sophisticated software and new approaches they integrated different kinds of measurements to interpret the complex data derived from the precious samples. Thousands of changes over the first week of life were found including in gene expression and components involved in immunity.
"Up to two thirds of newborn deaths can be prevented if effective health measures are provided at birth and during the first week of life. Of the 5.4 million under-five child deaths per year, about half occur during the neonatal period, meaning the first month of life. Knowledge about key developmental processes during our earliest days remains sparse, but this study plugs some of those crucial gaps. This work is particularly important for vaccine research. Newborns have very limited protection from infection in early life and there is an urgent need to optimise protective measures, including vaccines, used in this age group."
Beate Kampmann PhD, senior author, professor, Director of the Vaccine Centre, Paediatric Infection and Immunity, London School of Hygiene & Tropical Medicine.
Working closely with local communities, the research team recruited newborns in a health centre in The Gambia, West Africa. They took blood samples from each baby on the day of birth, and then again either on day one, three or seven. Blood samples were processed in the collaborating laboratories in Africa and North America, where researchers discovered dramatic molecular changes driven by development.
The findings were then validated in a second group of Australasian newborns. The two independent cohorts were found to have common, highly dynamic developmental trajectories, suggesting that the changes do not occur at random, but instead follow an age-specific pathway.
Prof Kampmann: "The MRC Unit in The Gambia has carried out important studies in newborns for a long time in order to optimize the use of vaccines. Given our excellent community relations and infrastructure, we were ready to partner with our collaborators to apply the new tools of systems biology to very small blood samples. We wanted to establish this work in a real world situation in order to gain insight into immune development in a setting where new interventions can have the biggest impact on newborn survival."
"Most infections occur early in life, newborns having the greatest susceptibility and the worst outcomes. Our findings allow us to ask about differences between populations and the impact of biomedical interventions, such as vaccines, on development. Deeper molecular insight into vaccine function in early life can develop into better infant vaccines. It is possible in a resource-poor setting to obtain small amounts of blood, process, ship, conduct systems biology assays and integrate those results - turning big data into knowledge."
Ofer Levy, Director, Precision Vaccines Program, Division of Infectious Diseases, Boston Children’s Hospital; Harvard Medical School, Boston; Broad Institute of MIT & Harvard, Cambridge, MA, USA; and a senior author on the paper.
Going forward, the EPIC team is currently investigating the impact of different vaccines on this early developmental trajectory by a larger cohort in The Gambia and Papua New Guinea. The authors acknowledge there are limitations to their study including validation in larger cohorts and increasing functional insights into the discovered pathways.
Abstract
Systems biology can unravel complex biology but has not been extensively applied to human newborns, a group highly vulnerable to a wide range of diseases. We optimized methods to extract transcriptomic, proteomic, metabolomic, cytokine/chemokine, and single cell immune phenotyping data from <1 ml of blood, a volume readily obtained from newborns. Indexing to baseline and applying innovative integrative computational methods reveals dramatic changes along a remarkably stable developmental trajectory over the first week of life. This is most evident in changes of interferon and complement pathways, as well as neutrophil associated signaling. Validated across two independent cohorts of newborns from West Africa and Australasia, a robust and common trajectory emerges, suggesting a purposeful rather than random developmental path. Systems biology and innovative data integration can provide fresh insights into the molecular ontogeny of the first week of life, a dynamic developmental phase that is key for health and disease.
Authors
Amy H. Lee, Casey P. Shannon, Nelly Amenyogbe, Tue B. Bennike, Joann Diray-Arce, Olubukola T. Idoko, Erin E. Gill, Rym Ben-Othman, William S. Pomat, Simon D. van Haren, Kim-Anh Lê Cao, Momoudou Cox, Alansana Darboe, Reza Falsafi, Davide Ferrari, Daniel J. Harbeson, Daniel He, Cai Bing, Samuel J. Hinshaw, Jorjoh Ndure, Jainaba Njie-Jobe, Matthew A. Pettengill, Peter C. Richmond, Rebecca Ford, Gerard Saleu, Geraldine Masiria, John Paul Matlam, Wendy Kirarock, Elishia Roberts, Mehrnoush Malek, Guzmán Sanchez-Schmitz, Amrit Singh, Asimenia Angelidou, Kinga K. Smolen, The EPIC Consortium, Ryan R. Brinkman, Al Ozonoff, Robert E. W. Hancock, Anita H. J. van den Biggelaar, Hanno Steen, Scott J. Tebbutt, Beate Kampmann, Ofer Levy and Tobias R. Kollmann.
Competing interests
O.L. is a named inventor on patents regarding bactericidal/permeability increasing protein (BPI), including “Therapeutic uses of BPI protein products in BPI-deficient humans” (WO2000059531A3) and “BPI and its congeners as radiation mitigators and radiation protectors” (WO2012138839A1). R.R.B. has ownership interest in Cytapex Bioinformatics Inc. The remaining authors declare no competing interests.
Acknowledgements
We would like to thank all the participants and their parents for their time and willingness to support this study. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health as part of the Human Immunology Project consortium under 5U19AI118608-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. T.R.K.’s laboratory is supported by a Michael Smith Foundation for Health Research Career Investigator Award. O.L.’s laboratory is supported by the following U.S. National Institutes of Health (NIH)/National Institutes of Allergy and Infectious Diseases (NIAID) awards: Molecular Mechanisms of Combination Adjuvants (1U01AI124284-01), Adjuvant Discovery Program Contract No. HHSN272201400052C and Human Immunology Project Consortium (U19AI118608) as well as an internal Boston Children’s Hospital award to the Precision Vaccines Program. B.K. is supported by grants from the MRC/UKRI (MC_UP_A900/1122, MC_UP_A900/115, MR/R005990/1), and the additional field team and laboratory staff at the MRC Unit in The Gambia. Recruitment of the cohort of newborns in Papua New Guinea was funded by seed funding awarded to A.H.J.v.d.B from the Wesfarmers Centre of Vaccines and Infectious Diseases, Telethon Kids Institute. The work in R.E.W.H.’s lab was initially supported by the Canadian Institutes for Health Research grant # FDN-154287 and he holds a Canada Research Chair in Health and Genomics and a UBC Killam Professorship. R.R.B.’s laboratory is supported by an award from Natural Sciences and Engineering Research Council of Canada. The Lundbeck Foundation (R181-2014-3372), The Carlsberg Foundation (CF14-0561), and A.P. Møller Foundation are acknowledged for grants enabling T.B.B.’s work. K.-A.L.C. is supported in part by the National Health and Medical Research Council (NHMRC) Career Development fellowship (GNT1087415). We gratefully acknowledge the support from Drs. Gary Fleisher, Michael Wessels and Ken Kraft as well as Maria Crenshaw, Mark Liu, Kerry McEnaney and Diana Vo (all BCH); Susan Farmer, Manish Sadarangani, Aaron Liu, Gordean Bjornson (all UBC). The Expanded Program on Immunization Consortium (EPIC) contributed collectively to this study. EPIC is an association of academic centers partnering to conduct systems biology studies in newborns and infants, comprised of the investigators listed above at Boston Children’s Hospital (BCH), University of British Columbia (UBC), Medical Research Council Unit The Gambia (MRCG), Université libre de Bruxelles, Telethon Kids Institute and University of Western Australia, and the Papua New Guinea Institute for Medical Research (PNG-IMR).
Funding: Chen Zhao was supported by the program of China Scholarship Council (201406990026) while carrying out the study. Professor Kathryn Abel is an NIHR senior Investigator and is funded by a European Research Council Consolidator award.
This study was funded through core grants from the Medical Research Council and the National Institutes of Health.
Return to top of page
| |
|
Mar 13, 2019 Fetal Timeline Maternal Timeline News
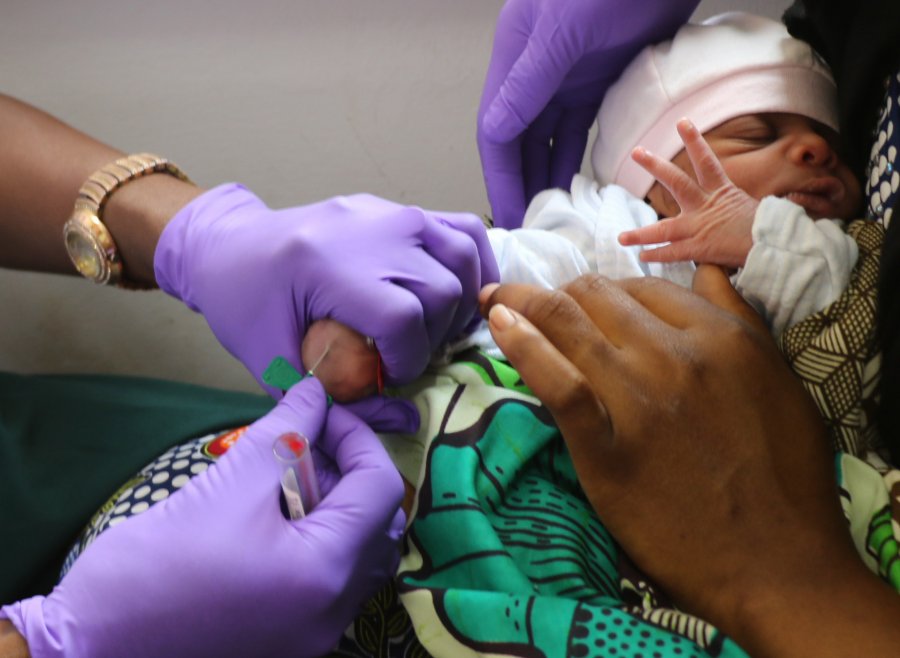 Newborn babies are the most vulnerable population when it comes to infectious disease. Establishing key pathways in early development could help measure the impact of factors such as diet, disease and maternal health, as well as which key interventions, like vaccines, have the most impact. Image: London School of Hygiene & Tropical Medicine.
|



