|
|
Developmental Biology - Blood Stem Cells
Squishing Blood Stem Cells
Squishing blood stem cells could help in harvesting them for transplants...
Scientists at Emory University's Winship Cancer Institute and the Children's Healthcare of Atlanta, Pediatric Technology Center - have found that altering the stiffness of blood-forming stem cells, might possibly make it easier to use them as stem cell-based transplants.
Temporary stem cell squishiness could help move blood-forming stem cells out of bone marrow and into blood circulation. But, researchers also found these same cells need to be stiff in order to stay put while replenishing blood and the immune system. Their research results were published March 14 in the journal Cell Stem Cell.
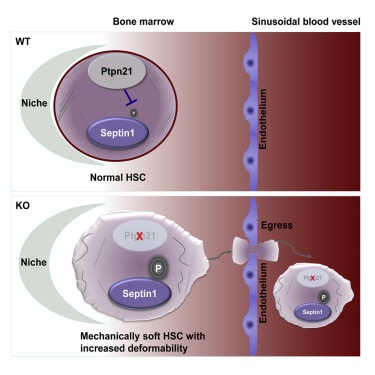 How stiff or squishy cells are, is important for keeping blood-forming stem cells
How stiff or squishy cells are, is important for keeping blood-forming stem cells
in bone marrow niches. This preserves their long-term repopulation capability.
Bone marrow transplants are a treatment strategy for some cancers, but usually don't involve physically extracting bone marrow. Doctors use the drug (G-CSF) to encourage blood-forming stem cells to leave bone marrow and enter the blood stream, generally producing a high yield. However, for about a third of bone marrow transplant patients, stem cell mobilization is insufficient. Cheng-Kui Qu MD PhD, professor of pediatrics at Emory University School of Medicine, explains that one of their experiments was a proof-of-concept strategy to supplement conventional stem cell extraction approaches.
Qu and colleagues studied the enzyme Ptpn21, which is highly expressed in blood stem cells and helps reshape a cell's internal skeleton. For the purposes of this study, the scientists generated mice without Ptpn21. They found in the bone marrow of these mutant mice, there were fewer stem and early progenitor cells. In addition, blood-forming stem cells tended to locate twice as far away from bone marrow niches - where they usually are found.
The mutant mice were very sensitive to chemotherapy drugs, making it easier to induce blood stem cells to move out of bone marrow niches. This was perhaps due to deformation of the cell wall as blood stem cells from mutant mice more easily squeezed through narrow pores.
Qu next approached Wilbur Lam and Todd Sulchek, biomedical engineers and experts on the mechanical characteristics of cells, who were able to determine that Ptpn21-mutant cells are indeed squishier. Qu's lab then performed experiments to make cells lacking Ptpn21 stiff again by manipulating another protein, Septin1. They were able to show that treating normal mice with blebbistatin, which interferes with a cell's internal skeleton, also mobilizes stem cells to leave bone niches. But, he cautions that blebbistatin may also have other systemic affects on mice.
"Our findings are that normal blood-forming stem cells are stiffer and less deformable than differentiated blood cells. This helps us better understand the pathogenesis of blood disorders associated with loss of stem cell quiescence. However, our findings suggest that cell biomechanics can be leveraged to improve current mobilization regimens for stem cell-based therapy."
Cheng-Kui Qu MD PhD, Professor of Pediatrics, Emory University School of Medicine; Division of Hematology/Oncology, Department of Pediatrics; Aflac Cancer and Blood Disorders Center, Winship Cancer Institute; Children’s Healthcare of Atlanta, Emory University, Atlanta, Georgia, USA.
Highlights
• Normal HSCs are stiffer and less deformable than mature blood cells
• Ptpn21 loss decreases cell stiffness and increases mobility/deformability in HSCs
• Deletion of Ptpn21 results in HSC egress due to impaired retention in the niche
• Ptpn21 modulates cellular mechanics by dephosphorylation of Septin1 (Tyr 246)
Summary
Hematopoietic stem cell (HSC) quiescence is a tightly regulated process crucial for hematopoietic regeneration, which requires a healthy and supportive microenvironmental niche within the bone marrow (BM). Here, we show that deletion of Ptpn21, a protein tyrosine phosphatase highly expressed in HSCs, induces stem cell egress from the niche due to impaired retention within the BM. Ptpn21 -/- HSCs exhibit enhanced mobility, decreased quiescence, increased apoptosis, and defective reconstitution capacity. Ptpn21 deletion also decreased HSC stiffness and increased physical deformability, in part by dephosphorylating Spetin1 (Tyr 246), a poorly described component of the cytoskeleton. Elevated phosphorylation of Spetin1 in Ptpn21 -/- cells impaired cytoskeletal remodeling, contributed to cortical instability, and decreased cell rigidity. Collectively, these findings show that Ptpn21 maintains cellular mechanics, which is correlated with its important functions in HSC niche retention and preservation of hematopoietic regeneration capacity.
Authors
Fang Ni, Wen-Mei Yu, Xinyi Wang, Meredith E. Fay, Katherine M. Young, Yongzhi Qiu, Wilbur A. Lam, Todd A. Sulchek, Tao Cheng, David T. Scadden an Cheng-Kui Qu.
The authors declare no conflict of interest.
Acknowledgements
Lam is a clinical hematologist-bioengineer at the Aflac Cancer and Blood Disorders Center of Children's Healthcare of Atlanta, associate professor of pediatrics at Emory University School of Medicine and a faculty member in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory. Sulchek is associate professor of mechanical engineering at Georgia Tech.
Research in Qu's lab is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (DK092722) and the National Heart Lung and Blood Institute (HL130995).
Return to top of page
| |
|
Mar 29 2019 Fetal Timeline Maternal Timeline News
 How deformable cells are, and thus how stiff or squishy they are, plays an important role in retaining blood-forming stem cells in their marrow niches and thus preserving their long-term repopulation capabilities. CREDIT: From Ni et al Cell Stem Cell (2019)
|




