|
|
Developmental Biology - Eyes
Assisting Preemies With Vision Problems
Light therapy may help redeem preemies loss of vision....
Scientists have discovered a light-dependent molecular pathway that regulates how blood vessels develop in the eye. The findings in Nature Cell Biology suggest it may be possible to use light therapy to help premature infants whose eyes are still developing, according to researchers at Cincinnati Children's Hospital Medical Center.
Called the opsin 5-dopamine pathway, this new molecular process helps blood-vessels develop in the eye appropriately balanced in preparation for visual function. The process can be thrown out of balance in medically fragile premature babies.
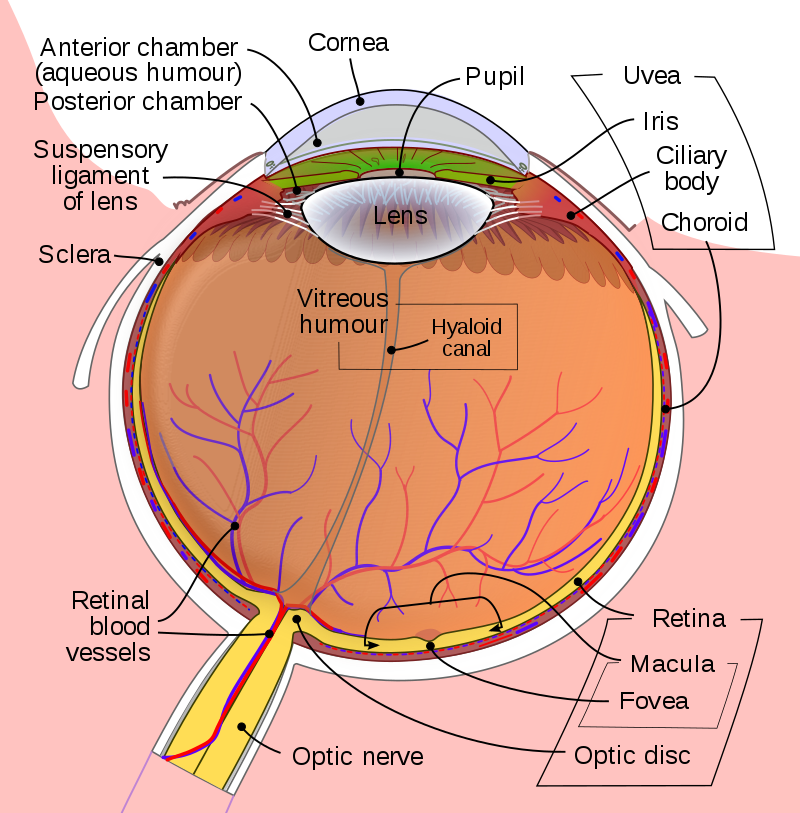
The Hyaloid Canal extends from the lens to the optic nerve. Wikipedia
"Our study indicates opsin 5-dopamine pathway is probably part of a light-dependent disease process for conditions like myopia (severe near-sightedness), now a worldwide epidemic," explains Richard A. Lang PhD, director of the Visual Systems Group at Cincinnati Children's and study senior author. "It raises the interesting possibility that we might be able to use light exposure to treat conditions like retinopathy of prematurity after a premature infant is born — or in people with myopia."
According to Lang, opsin 5 is highly conserved across species in evolution, enhancing it's relevance to humans.
Research in mice demonstrates how the postnatal eye depends on light responses controlled by opsin 5 - a protein expressed in special photoreceptor cells in the retina.
Opsin 5 and the neurotransmitter dopamine — which promotes blood vessels to return to a less developed state (regress) — work in unison to balance blood vessel development in the eye.
To show what would happen without the balancing influence of opsin 5, researchers genetically modified mice without it in the retina. This increased levels of dopamine in the clear, gel-like substance in the eye (the vitreous) causing hyaloid blood vessels to quickly regress, hindering eye development.
Shedding Light on the Problem
To test the influence of light stimulation, researchers used violet colored 380 nanometer light which activated opsin 5 signaling. This reduced dopamine levels in the eye and produced other molecular changes that helped restore timing cues needed to balance vascular development.
Previous studies also suggest violet light and dopamine are key regulators in eye development. Although the current study requires additional research to become clinically relevant to humans, it supports balanced coordination of opsin 5 to dopamine is relevant for healthy eye development in baby mice, and likely human babies.
Abstract
During mouse postnatal eye development, the embryonic hyaloid vascular network regresses from the vitreous as an adaption for high-acuity vision. This process occurs with precisely controlled timing. Here, we show that opsin 5 (OPN5; also known as neuropsin)-dependent retinal light responses regulate vascular development in the postnatal eye. In Opn5-null mice, hyaloid vessels regress precociously. We demonstrate that 380-nm light stimulation via OPN5 and VGAT (the vesicular GABA/glycine transporter) in retinal ganglion cells enhances the activity of inner retinal DAT (also known as SLC6A3; a dopamine reuptake transporter) and thus suppresses vitreal dopamine. In turn, dopamine acts directly on hyaloid vascular endothelial cells to suppress the activity of vascular endothelial growth factor receptor 2 (VEGFR2) and promote hyaloid vessel regression. With OPN5 loss of function, the vitreous dopamine level is elevated and results in premature hyaloid regression. These investigations identify violet light as a developmental timing cue that, via an OPN5–dopamine pathway, regulates optic axis clearance in preparation for visual function.
Authors
Minh-Thanh T. Nguyen, Shruti Vemaraju, Gowri Nayak, Yoshinobu Odaka, Ethan D. Buhr, Nuria Alonzo, Uyen Tran, Matthew Batie, Brian A. Upton, Martin Darvas, Zbynek Kozmik, Sujata Rao, Rashmi S. Hegde, P. Michael Iuvone, Russell N. Van Gelder and Richard A. Lang.
Acknowledgements
The authors thank P. Speeg (Lang lab) for excellent mouse colony management, L. Sankaran and P. Lyuboslavsky (Iuvone lab) for technical assistance, and D. Bredl and D. Copenhagen (UCSF) for providing tissue samples from the Drd2-eGFP mice. We also thank Y. Chen and Y.-C. Hu of the CCHMC Transgenic Animal and Genome Editing Core Facility for generating genetically modified mouse lines. This work was supported by NIH R01 GM124246 to E.D.B., NIH R01EY026921 to R.N.V.G., NIH P30EY001730 to the University of Washington, the Mark J. Daily, MD Research Fund to the University of Washington, and unrestricted grants to the University of Washington and Emory University Department of Ophthalmology from Research to Prevent Blindness. This work was also supported by NIH grants R01 EY027077 (R.A.L. and S.R.), R01 EY027711 (P.M.I. and R.A.L.), R01 EY022917 (R.S.H.) and R01 EY004864 (P.M.I.), by funds from the Goldman Chair of the Abrahamson Pediatric Eye Institute at Cincinnati Children’s Hospital Medical Center and by grant BIOCEV-CZ.1.05/1.1.00/02.0109 (Z.K.). This work was supported by NIH grant 2T32GM063483, which supports the UCCOM/CCHMC Medical Scientist Training Program.
Return to top of page
| |
|
Apr 2 2019 Fetal Timeline Maternal Timeline News
 Microscopic image of light-sensitive opsin proteins in the retinal ganglia nerve fibers of a neonatal mouse eye. This animal model helped scientists identify the role opsin proteins play in the developing neonatal eye. Researchers believe light-based therapies may prevent (or treat) eye diseases like myopia and retinopathy of prematurity. Image: Cincinnati Children's Hospital Medical Center
|




