|
|
Developmental Biology - Cell Structures and Disease
Two Enzymes May Trigger Muscle & Brain Disorders
St. Jude Children's research finds 2 enzymes that help break down "stress granules" can also fail...
St. Jude Children's Research Hospital has identified two enzymes, ULK1 and ULK2, as key in breaking down cell structures called stress granules which arise to protect the cell, can also create toxic protein buildup and kill muscle and brain cells if they fail to disperse those granules completely.
Three diseases related to ULK1 and ULK2 buildup:
(1) Inclusion body myopathy (IBM)
IBM causes weakness in arm and leg muscles.
(2) Amyotrophic Lateral Sclerosis (ALS)
Also known as Lou Gehrig's disease, causes paralysis due to the death of nerve cells controlling voluntary muscles.
(3) FrontoTemporal Dementia (FTD).
A form of dementia that damages areas of the brain associated with personality, behavior and language.
Led by St. Jude researcher Mondira Kundu MD PhD, an associate member of the St. Jude Department of Pathology, the team published their findings in the journal Molecular Cell.
Stress granules are biological "storm shelters" that temporarily protect genetic molecules and proteins when a cell's health is under threat from heat, chemicals or infection.
Although stress granules normally disassemble when the source of stress is removed, gene mutations can cause malfunctions that keep them around. For example, researchers found a gene mutation called VC which is a key activator of ULK1 and ULK2 production.
Researchers believe in the future, drugs can be made to boost ULK1 and ULK2 in order to help treat the pathologies of IBM, ALS and FTD.
Highlights
• Ulk1 and Ulk2 deficiency causes IBM-like disease with TDP-43+ and ubiquitin+ pathology
• ULK1 and ULK2 promote resolution of stress granules in an autophagy-independent manner
• ULK1 and ULK2 localize to stress granules and interact with the AAA+ ATPase VCP
• Phosphorylation of VCP by ULK1 and ULK2 is key to efficient disassembly of stress granules
Summary
Disturbances in autophagy and stress granule dynamics have been implicated as potential mechanisms underlying inclusion body myopathy (IBM) and related disorders. Yet the roles of core autophagy proteins in IBM and stress granule dynamics remain poorly characterized. Here, we demonstrate that disrupted expression of the core autophagy proteins ULK1 and ULK2 in mice causes a vacuolar myopathy with ubiquitin and TDP-43–positive inclusions; this myopathy is similar to that caused by VCP/p97 mutations, the most common cause of familial IBM. Mechanistically, we show that ULK1/2 localize to stress granules and phosphorylate VCP, thereby increasing VCP’s activity and ability to disassemble stress granules. These data suggest that VCP dysregulation and defective stress granule disassembly contribute to IBM-like disease in Ulk1/2-deficient mice. In addition, stress granule disassembly is accelerated by an ULK1/2 agonist, suggesting ULK1/2 as targets for exploiting the higher-order regulation of stress granules for therapeutic intervention of IBM and related disorders.
Authors
Bo Wang, Brian A.Maxwell, Joung Hyuck Joo, Young dae Gwon, James Messing, Ashutosh Mishra, Timothy I. Shaw, Amber L. Ward, Honghu Quan, Sadie Miki Sakurada Shondra M. Pruett-Miller, Tulio Bertorini, PeterVoge, Hong Joo Kim, Junmin Peng, J. Paul Taylor, Mondira Kundu.
Acknowledgements
The research was supported by the National Institutes of Health (R01 MH115058, HL114697, R01 GM114260, R35 NS097974), the Robert Packard Center for ALS Research and ALSAC, the fundraising and awareness organization of St. Jude.
Return to top of page
| |
|
Apr 11 2019 Fetal Timeline Maternal Timeline News
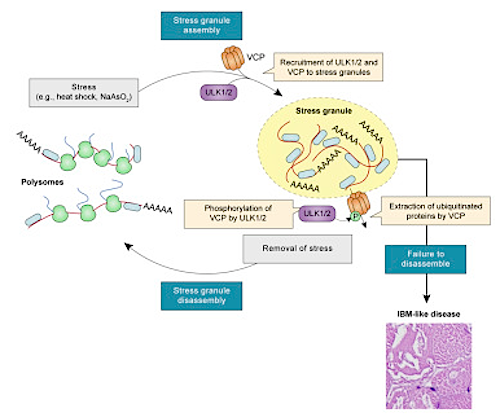 Stress granules arise to protect a cell's molecules and proteins from toxic elements. However, they can become toxic and kill muscle and brain cells if they fail to disperse after completion of their role. Credit: St. Jude Children's Research Hospital.
|



