|
|
Developmental Biology - Stem Cells
Hair Follicle Stem Cells Repair Damaged Neurons
Hair follicle stem cells in mice produce pigment cells that can regenerate the myelin sheath coating neurons...
A subset of stem cells in hair follicles of mice have the potential to regenerate the coating insulating neurons, reports Thomas Hornyak MD, United States Department of Veterans Affairs; Maryland Health Care System and the University of Maryland School of Medicine, along with his colleagues in a new study published 24th April in PLOS Genetics. Their study offers new therapeutic options for treating certain neurodegenerative diseases.
Hair and skin take on varying shades of red, brown, black and yellow due to pigment cells called melanocytes. Melanocytes originate in the embryo from cells called neural crest cells — which can also give rise to neurons and their glial cells.
Previously, Hornyak and colleagues identified two different pockets of stem cells that create melanocytes inside mature hair follicles.
In the current study, they show how these two groups of melanocyte stem cells can be identified and then separated based on whether they are coated in a glycoprotein called CD34. This surface molecule is also present on other types of stem cells, including blood stem cells. Using hair follicles from mice, they were able to isolate these two populations of melanocyte stem cells and grow them in culture.
Researchers were surprised to see that cells carrying CD34 turned into glial cells.
In the nervous system, glial cells coat neurons with fatty insulation called myelin, which increases the speed at which nerve impulses travel. Furthermore, researchers discovered that CD34-positive stem cells can regenerate myelin on neurons, in both cells in culture and when injected into mice carrying a genetic defect preventing them from forming myelin sheaths.
These new findings suggest that CD34-positive melanocyte stem cells in hair follicles retain some of their embryonic flexibility. If similar populations exist in human hair follicles, they could be tapped to develop into new treatments for nerve injuries and demyelinating diseases, such as multiple sclerosis.
"In the future, we plan to continue research in this area to determine whether these cells can enhance functional recovery from neuronal injury — and leverage genome-wide information in the current study to identify similar cells in human skin."
Dr. Thomas Hornyak MD PhD, Associate Professor of Dermatology; University of Maryland Medical Center; Member of UM Faculty Physicians, Inc.; Baltimore, Maryland, USA.
Abstract
Melanocyte stem cells (McSCs) are undifferentiated melanocytic cells of the mammalian hair follicle (HF) responsible for recurrent generation of a large number of differentiated melanocytes during each HF cycle. HF McSCs reside in both the CD34+ bulge/lower permanent portion (LPP) and the CD34- secondary hair germ (SHG) regions of the HF during telogen. Using Dct-H2BGFP mice, we separate bulge/LPP and SHG McSCs using FACS with GFP and anti-CD34 to show that these two subsets of McSCs are functionally distinct. Genome-wide expression profiling results support the distinct nature of these populations, with CD34- McSCs exhibiting higher expression of melanocyte differentiation genes and with CD34+ McSCs demonstrating a profile more consistent with a neural crest stem cell. In culture and in vivo, CD34- McSCs regenerate pigmentation more efficiently whereas CD34+ McSCs selectively exhibit the ability to myelinate neurons. CD34+ McSCs, and their counterparts in human skin, may be useful for myelinating neurons in vivo, leading to new therapeutic opportunities for demyelinating diseases and traumatic nerve injury.
Author summary
The hair follicle (HF) undergoes three different stages, anagen, catagen, and telogen during each hair cycle. In anagen, melanocyte stem cells (McSCs) give rise to differentiated melanocytes which are responsible for coloration of hair. In catagen, melanocytes undergo apoptosis while McSCs are retained. In the resting telogen HF, McSCs are identified as non-proliferating and quiescent populations. Interestingly, in a mouse model, we identified McSCs in both CD34+ bulge and CD34- secondary hair germ (SHG) compartments of telogen HFs. In this study, we separated and characterized McSC subpopulations from these two distinct compartments of telogen HFs. Using Dct-H2BGFP mice, bulge and SHG McSCs were separated using CD34. Based on genomic approaches and functional assays we found that CD34- McSCs (SHG) are primed for melanocyte differentiation and CD34+ McSCs (bulge) exhibit broader neural crest stem cell properties and demonstrate ability to differentiate into glia and myelinate neurons. Our results thus reveal functional heterogeneity of McSC subtypes.
Authors
Sandeep S. Joshi, Bishal Tandukar, Li Pan, Jennifer M. Huang, Ferenc Livak, Barbara J. Smith, Theresa Hodges, Anup A. Mahurkar and Thomas J. Hornyak.
Acknowledgements
Funding: This research was supported in part by NIH/NIAMS R01 grant 1R01AR064810, United States Department of Health & Human Services; VA Merit Award BX002582, Biomedical Laboratory Research & Development, Office of Research & Development, United States Department of Veterans Affairs; Dean’s Office funds, University of Maryland School of Medicine; and the Baltimore Research and Education Foundation, VA Maryland Health Care System. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors thank Elaine Fuchs for permission to use the TRE-H2BGFP mouse strain in this study. Flow cytometry analyses and cell sorting were performed at the University of Maryland Marlene and Stuart Greenebaum Cancer Center Flow Cytometry Shared Service.
Return to top of page
| |
|
Apr 25 2019 Fetal Timeline Maternal Timeline News
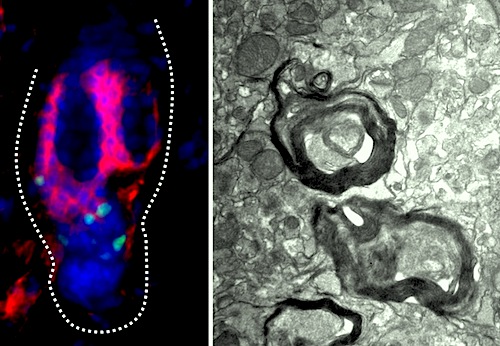 On the LEFT are (GREEN) melanocyte stem cells from the telogen, resting phase, of mouse hair follicle inside (RED) CD34-positive bulge. These cells differentiate to form dense (RIGHT) myelin sheaths in brain of myelin-deficient mice. Image: Sandeep Joshi, University of Maryland School of Medicine
|



