|
|
Developmental Biology - Birth Defects
How Thalidomide Can Affect Brain Size
A molecular basis for thalidomide brain malformations...
In the late 1950s, thalidomide was prescribed as a treatment for afflictions ranging from anxiety to morning sickness.
By the early 1960s, research found it the primary cause of in utero birth defects, ranging from limb malformation to deafness and/or blindness.
Handa's group at Tokyo Medical University identified that thalidomide selectively binds to a protein called cereblon (CRBN), dysfunction of which reportedly results in mental retardation and epilepsy in humans.
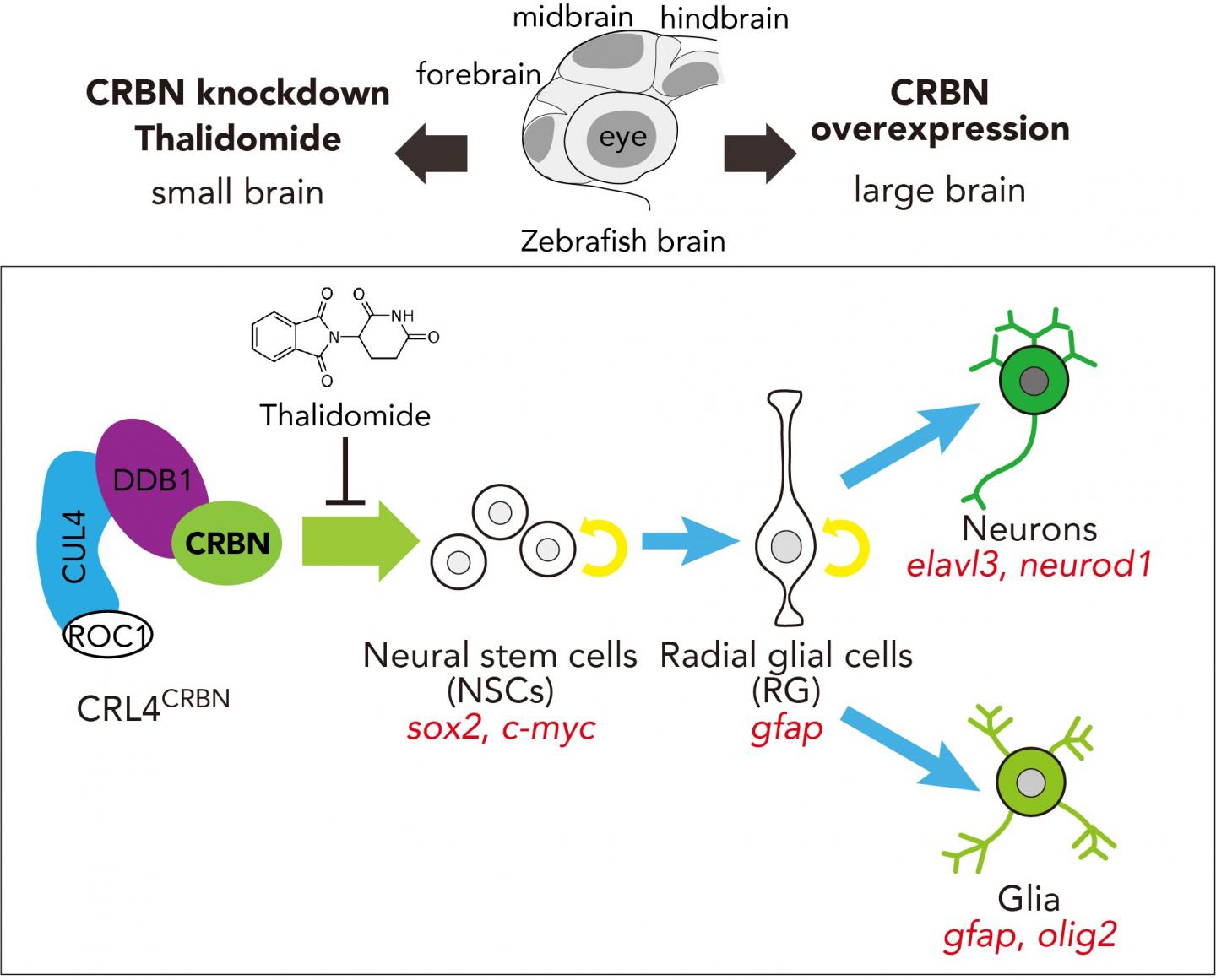
To determine the mechanisms involved in CRBN dysfunction and its downstream effects, Handa, Yamaguchi (Tokyo Tech), and their teams studied its role in a common vertebrate animal model (zebrafish), finding that the CRBN gene encodes a protein involved in the survival and differentiation of neural stem cells (NSCs). During the embryonic stage, NSCs transform into neurons and glial cells, which are directly involved in brain development.
Their findings, appearing in iScience, showed that decreased levels of available CRBN protein resulted in smaller brains in zebrafish embryos, whereas increasing its availability resulted in larger brains.
 Normal CRBN activity promotes NSC proliferation and differentiation into neurons and glial cells; however, thalidomide binding to CRBN inhibits this activity, resulting in NSC death, smaller-sized brains, and dysfunctional organ, limb, and/or mental development. CREDIT ©Tokyo Tech
Normal CRBN activity promotes NSC proliferation and differentiation into neurons and glial cells; however, thalidomide binding to CRBN inhibits this activity, resulting in NSC death, smaller-sized brains, and dysfunctional organ, limb, and/or mental development. CREDIT ©Tokyo Tech
Importantly, parallel experiments in zebrafish embryos with normal levels of CRBN revealed that treatment with thalidomide showed results similar to those observed in embryos with depleted levels of CRBN. Handa commented that these findings "suggest that CRBN selectively binds thalidomide, which alters the CRBN functions involved in promoting normal embryonic development".
Specifically, CRBN is a member of a group of molecules responsible for allowing the creation of neurons and glial cells, the latter of which are responsible for neuron proliferation and protection, from NSCs during the early stages of brain development. The authors describe a process by which inhibition of CRBN function results in the premature death of these NSCs, thereby preventing the creation of cells required for normal brain and organ development.
Importantly, the authors' findings suggest a role for the CRBN gene in regulating brain size.
"This suggests potential opportunities to develop therapeutic strategies for afflictions associated with macrocephaly related to excessive brain size, such as autism spectrum disorder," explains Yamaguchi.
Thalidomide derivatives can be developed to control unchecked proliferation of NSCs in order to address such afflictions.
Highlights
• CRBN is a determinant of head and brain size during zebrafish development
• Thalidomide causes a reduction in head and brain size by binding to CRBN
• CRBN prevents apoptosis and promotes NSC proliferation during brain development
• crbn overexpression results in a concomitant increase in neurons and glial cells
Summary
Thalidomide is a teratogen that causes multiple malformations in the developing baby through its interaction with cereblon (CRBN), a substrate receptor subunit of the CRL4 E3 ubiquitin ligase complex. CRBN was originally reported as a gene associated with autosomal recessive non-syndromic mild mental retardation. However, the function of CRBN during brain development remains largely unknown. Here we demonstrate that CRBN promotes brain development by facilitating the proliferation of neural stem cells (NSCs). Knockdown of CRBN in zebrafish embryos impaired brain development and led to small brains, as did treatment with thalidomide. By contrast, overexpression of CRBN resulted in enlarged brains, leading to the expansion of NSC regions and increased cell proliferation in the early brain field and an expanded expression of brain region-specific genes and neural and glial marker genes. These results demonstrate that CRBN functions in the determination of brain size by regulating the proliferation of NSCs during development.
Authors
Hideki Ando, Tomomi Sato, Takumi Ito, Junichi Yamamoto, Satoshi Sakamoto, Nobuhiro Nitta, Tomoko Asatsuma-Okumura, Nobuyuki Shimizu, Ryota Mizushima, Ichio Aoki, Takeshi Imai, Yuki Yamaguchi, Arnold J. Berk and Hiroshi Handa.
Acknowledgments
The authors thank Dr. Ichiro Masai (OIST) for technical support and helpful discussions. We thank Kentaro Hotta, Masahiko Manabe, Masayo Akiyama, and Yurika Kubo for technical assistance. We thank the Zebrafish International Resource Center for technical support. Tg(elavl3:Kaede) was provided from Zebrafish National BioResource Project. Tg(her5PAC:egfp) was kindly provided by Laure Bally-Cuif. This work was supported by JSPS KAKENHI grant numbers 17H06112 (to H.H. and Y.Y.), 15H04288 (to H.A.), 18K09271 (to T.S.), 17H04213 (to T. Ito), 18H05502 (to T. Ito), MEXT-Supported Program for the Strategic Research Foundation at Private Universities S1411011 (to H.H.), PRESTO, JST JPMJPR1531 (to T. Ito), and Research Grant for Geriatrics and Gerontology NCGG 28-25 (to T. Imai).
Return to top of page
| |
|
May 17 2019 Fetal Timeline Maternal Timeline News
|




