|
|
Developmental Biology - Stem Cells
All Immature Cells Can Become Stem Cells
New study disproves traditional stem cell development...
The study reveals that the destiny of intestinal cells is not predetermined, but instead determined by the cells' surroundings. The new knowledge may make it easier to manipulate stem cells for stem cell therapy. The results have just been published in Nature.
All cells in the fetal gut have the potential to develop into stem cells, a new study conducted at the Faculty of Health and Medical Sciences at the University of Copenhagen concludes. Researchers behind the work say that development of immature intestinal cells - contrary to previous assumptions - is not predetermined, but affected by the cells' immediate surroundings in the intestines.
This discovery may ease the path to effective stem cell therapy, suggests Associate Professor Kim Jensen from the Biotech Research & Innovation Centre (BRIC) and the Novo Nordisk Foundation Center for Stem Cell Biology (DanStem).
"We used to believe that a cell's potential for becoming a stem cell was predetermined, but our new results show that all immature cells have the same probability for becoming stem cells in the fully developed organ. In principle, it is simply a matter of being in the right place at the right time. Here signals from the cells' surroundings determine their fate. If we are able to identify the signals that are necessary for the immature cell to develop into a stem cell, it will be easier for us to manipulate cells in the wanted direction."
Throughout life the organs in the body are maintained by stem cells, which are also able to repair minor tissue damage. A better understanding of the factors that determine whether or not an immature cell develops into a stem cell may therefore be useful in the development of stem cells for therapy and transplantation.
"We have gained greater insight into the mechanisms through which cells in the immature intestines develop into stem cells. Hopefully we are able to use this knowledge to improve treatment of non-healing wounds, e.g. in the intestines. So far, though, all we can say for sure is that cells in the gastrointestinal tract have these characteristics. However, we do believe this is a general phenomenon in fetal organ development."
The surprising findings are the result of a search for understanding of what controls the destiny of intestinal stem cells. Postdoc Jordi Guiu developed a method for monitoring the development of the individual intestinal cells. By introducing luminescent proteins into the cells he could, using advanced microscopy, monitor the development of the individual cells.
After the initial tests, cells that researchers previously believed to be fetal stem cells were only able to explain a fraction of the growth of the intestines during fetal development. Therefore, they established a collaboration with mathematical experts at the University of Cambridge. Studying the data more closely together, they arrived at the surprising hypothesis that all intestinal cells may have the same chance of becoming stem cells. Subsequent tests proved the hypothesis.
"The next step is to determine precisely which signals are necessary for immature cells to develop into the kind of stem cells we need. This is one of our research focusses."
Kim B. Jensen PhD, Biotech Research and Innovation Centre (BRIC), Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Novo Nordisk Foundation Center for Stem Cell Research, University of Copenhagen, Copenhagen, Denmark.
Throughout life stem cells help maintain the organs in the body and repair damaged tissue. However, the stem cells found in the body can only renew and repair minor tissue damage.
Using stem cell transplantation and therapy it is possible to supplement the body's own cells with new, healthy stem cells that can help repair or replace damaged tissue.
Abstract
Adult intestinal stem cells are located at the bottom of crypts of Lieberkühn, where they express markers such as LGR51,2 and fuel the constant replenishment of the intestinal epithelium1. Although fetal LGR5-expressing cells can give rise to adult intestinal stem cells3,4, it remains unclear whether this population in the patterned epithelium represents unique intestinal stem-cell precursors. Here we show, using unbiased quantitative lineage-tracing approaches, biophysical modelling and intestinal transplantation, that all cells of the mouse intestinal epithelium—irrespective of their location and pattern of LGR5 expression in the fetal gut tube—contribute actively to the adult intestinal stem cell pool. Using 3D imaging, we find that during fetal development the villus undergoes gross remodelling and fission. This brings epithelial cells from the non-proliferative villus into the proliferative intervillus region, which enables them to contribute to the adult stem-cell niche. Our results demonstrate that large-scale remodelling of the intestinal wall and cell-fate specification are closely linked. Moreover, these findings provide a direct link between the observed plasticity and cellular reprogramming of differentiating cells in adult tissues following damage, revealing that stem-cell identity is an induced rather than a hardwired property.
Authors
Jordi Guiu, Edouard Hannezo, Shiro Yui, Samuel Demharter, Svetlana Ulyanchenko, Martti Maimets, Anne Jørgensen, Signe Perlman, Lene Lundvall, Linn Salto Mamsen, Agnete Larsen, Rasmus H. Olesen, Claus Yding Andersen, Lea Langhoff Thuesen, Kristine Juul Hare, Tune H. Pers, Konstantin Khodosevich, Benjamin D. Simons and Kim B. Jensen.
Acknowledgements
The authors thank members of the Jensen and Simons laboratories for comments and suggestion; H. Clevers (Rosa26-lsl-Confetti and Lgr5-eGFP-ires-creERT2), F. de Sauvage (Genentech) (Lgr5-iDTR-eGFP), A. McMahon (Krt20-T2A-creERT2) and G. Gu (Krt19-creERT) for gifts of mice; and Y. Antoku in Imaging core facilities at BRIC and the Center for Advanced Bioimaging at University of Copenhagen for experimental support. This work was supported by Lundbeck Foundation (R105-A9755 to K.B.J.; R190-2014-3904 to T.H.P.), the Novo Nordisk Foundation (NNF14OC0012927 to K.B.J. and NNF16OC0019920 to K.K.), the Carlsberg Foundation, EMBO Young Investigator programme (to K.B.J.), the Marie Curie fellowship programme (S.Y. and J.G.; 625238/FP7-PEOPLE-2013-IIF, 656099/H2020-MSCA-IF-2014) and the Wellcome Trust (098357/Z/12/Z to B.D.S., 110326/Z/15/Z to E.H.). B.D.S. also acknowledges funding from the Royal Society E. P. Abraham Research Professorship (RP\R1\180165). This project has received funding from the European Union’s Horizon 2020 research and innovation programme (grant agreements STEMHEALTH ERCCoG682665 and INTENS 668294 to K.B.J.). The Novo Nordisk Foundation Center for Stem Cell Biology and the Novo Nordisk Foundation Center for Basic Metabolic Research are supported by Novo Nordisk Foundation grants (NNF17CC0027852 and NNF18CC0034900, respectively).
The project was funded by the European Research Council, the Horizon 2020 research programme, the Lundbeck Foundation, the Novo Nordisk Foundation, the Carlsberg Foundation and the Marie Curie fellowship programme.
Return to top of page
| |
|
May 20 2019 Fetal Timeline Maternal Timeline News
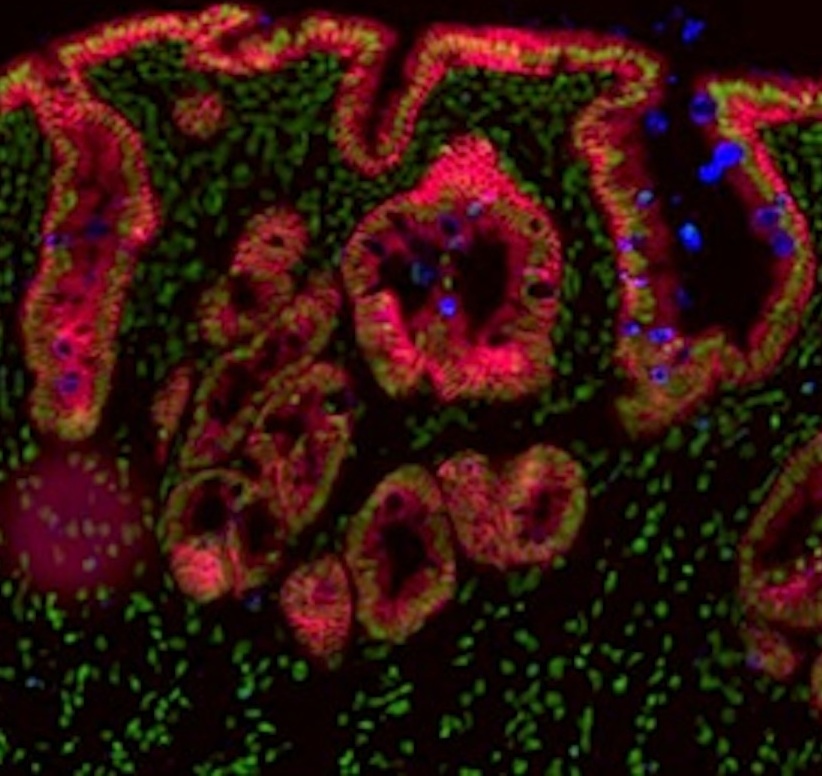 Fetal mouse gut showing involutions of gut lining with colored cells reflecting tissue differentiation. Credit: Faculty of Health and Medical Sciences; University of Copenhagen.
|



