|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
Developmental Biology - Cell Size What Controls Cell Size? Although researchers discovered the mechanisms in bacteria Escherichia coli (E. coli) and Bacillus subtilis (B. subtilis), they believe the process is applicable across many life forms. Jun adds that the research team, made up of biologists, physicists and engineers, cracked the adder case after years of attempting an array of investigative methods and experimental approaches. He continues: "Cell size homeostasis is a fundamental biological question and to our knowledge this is the first time we finally understand its mechanistic origin. We would not have been able to solve this with pure physics or pure biology. It was a very multi-disciplinary approach." They are now investigating whether the quantitative and mechanistic framework underlying adder applies to other models such as yeast and cancer cells. Highlights • The adder requires accumulation of division proteins to a threshold for division • The adder requires constant production of division proteins during cell elongation • In E. coli and B. subtilis, initiation and division are independently controlled • In E. coli and B. subtilis, cell division exclusively drives size homeostasis Summary Evolutionarily divergent bacteria share a common phenomenological strategy for cell-size homeostasis under steady-state conditions. In the presence of inherent physiological stochasticity, cells following this “adder” principle gradually return to their steady-state size by adding a constant volume between birth and division, regardless of their size at birth. However, the mechanism of the adder has been unknown despite intense efforts. In this work, we show that the adder is a direct consequence of two general processes in biology: (1) threshold—accumulation of initiators and precursors required for cell division to a respective fixed number—and (2) balanced biosynthesis—maintenance of their production proportional to volume growth. This mechanism is naturally robust to static growth inhibition but also allows us to “reprogram” cell-size homeostasis in a quantitatively predictive manner in both Gram-negative Escherichia coli and Gram-positive Bacillus subtilis. By generating dynamic oscillations in the concentration of the division protein FtsZ, we were able to oscillate cell size at division and systematically break the adder. In contrast, periodic induction of replication initiator protein DnaA caused oscillations in cell size at initiation but did not alter division size or the adder. Finally, we were able to restore the adder phenotype in slow-growing E. coli, the only known steady-state growth condition wherein E. coli significantly deviates from the adder, by repressing active degradation of division proteins. Together, these results show that cell division and replication initiation are independently controlled at the gene-expression level and that division processes exclusively drive cell-size homeostasis in bacteria. Authors Fangwei Si, Guillaume Le Treut, John T. Sauls, Stephen Vadia, Petra Anne Levin and Suckjoon Jun. Acknowledgements The authors are deeply grateful to Willie Donachie for invaluable discussions while completing this work. They thank Dongyang Li, Rodrigo Reyes-Lamothe, Tsutomu Katayama, Anders Løbner-Olesen, Harold Erickson, William Margolin, and Paul Wiggins for providing the strains. This work was supported by the Paul G. Allen Family Foundation, Pew Charitable Trust, NSF CAREER grant MCB-1253843, and NIH grants R01 GM118565-01 (to S.J.) and R35-400 GM127331 (to P.A.L.). Return to top of page | May 24 2019 Fetal Timeline Maternal Timeline News 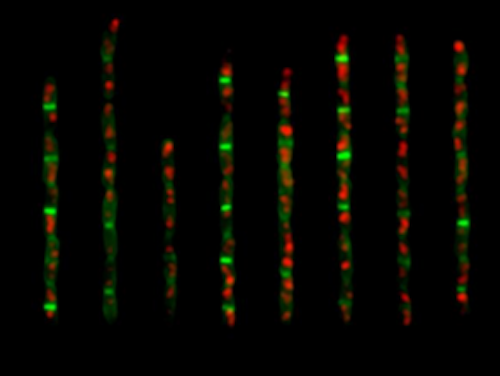 E. coli cells expressing fluorescent fusion proteins of the replisome in two colors. The replisome is a complex molecular machine that carries out replication of DNA - by first unwinding double stranded DNA into two single strands. Each of these single strands is now a new sequence of combined DNA. CREDIT Jun Lab, UC San Diego.
|
||||||||||||||||||||||||||||

