|
|
Developmental Biology - Down Syndrome
A Down Syndrome Treatment?
Rutgers-led team uses stem cell-based disease models to pinpoint Down syndrome gene link to impaired memory...
Targeting a key gene before birth could someday help lead to a treatment for Down syndrome by reversing abnormal embryonic brain development and improving cognitive function after birth, according to a Rutgers-led study.
Using stem cells that can turn into other cells, researchers developed two experimental models (1) a living 3D "organoid" model of the brain and (2) a mouse brain model with implanted human cells — to investigate early brain development linked to Down's. The study appears in Cell Stem Cell, and focused on human chromosome 21 gene OLIG2.
"Our results suggest the OLIG2 gene is potentially an excellent prenatal therapeutic target to reverse abnormal embryonic brain development, rebalance the two types of neurons in the brain — excitatory and inhibitory, as a healthy balance is critical — as well as improve postnatal cognitive function."
Peng Jiang PhD, Assistant Professor, Department of Cell Biology and Neuroscience, Rutgers University-New Brunswick, New Jersey, USA.
Usually, a baby is born with 46 chromosomes, but babies with Down syndrome have an extra copy of chromosome 21. That changes how a baby's body and brain develops, which can lead to mental and physical challenges, according to the U.S. Centers for Disease Control and Prevention. Down syndrome patients often develop early-onset Alzheimer's disease as well.
Down syndrome is the most common chromosomal condition diagnosed in the United States. It affects about one in 700 babies, about 6,000 infants are born with the condition each year.
Researchers collected skin cells from Down patients and genetically reprogrammed those cells into human-induced pluripotent stem cells (hiPSCs). Resembling embryonic stem cells, these special cells can develop into many different types of cells, including brain cells, during early life and growth and are useful tools for drug development and disease modeling, according to the National Institutes of Health.
Using brain cells derived from stem cells with an extra copy of chromosome 21, the scientists developed the 3D brain organoid model resembling the early developing human brain. They also developed the mouse brain model, using stem cell-derived human brain cells implanted into the mouse brains within 24 hours of the mice being born. They found that inhibitory neurons - which make our brain function smoothly - were overproduced in both models. The adult mice had impaired memory. They also found the OLIG2 gene is critical to those effects — inhibiting it led to improvements.
Reducing OLIG2 function normalizes interneuron production and behavioral deficits.
These results support that working with both brain organoids and a mouse brain model can be useful in studying neurodevelopmental disorders — such as autism spectrum disorder. Both tools may also help better explain mechanisms behind Alzheimer's disease.
Highlights
• Down syndrome (DS) brain organoids overproduce OLIG2+ progenitors
• DS brain organoids and chimeric mouse brains exhibit excessive interneuron production
• OLIG2 directly upregulates expression of transcriptional regulators of interneuron fate
• Reducing OLIG2 expression normalizes interneuron production and behavioral deficits
Summary
Down syndrome (DS) is a common neurodevelopmental disorder, and cognitive defects in DS patients may arise from imbalances in excitatory and inhibitory neurotransmission. Understanding the mechanisms underlying such imbalances may provide opportunities for therapeutic intervention. Here, we show that human induced pluripotent stem cells (hiPSCs) derived from DS patients overproduce OLIG2+ ventral forebrain neural progenitors. As a result, DS hiPSC-derived cerebral organoids excessively produce specific subclasses of GABAergic interneurons and cause impaired recognition memory in neuronal chimeric mice. Increased OLIG2 expression in DS cells directly upregulates interneuron lineage-determining transcription factors. shRNA-mediated knockdown of OLIG2 largely reverses abnormal gene expression in early-stage DS neural progenitors, reduces interneuron production in DS organoids and chimeric mouse brains, and improves behavioral deficits in DS chimeric mice. Thus, c in DS patients.
Authors
RanjieXu, Andrew T. Brawner, Shenglan Li, Jing-Jing Liu, Hyosung Kim, Haipeng Xue, Zhiping P.Pang, Woo-Yang Kim, Ronald P.Hart, Ying Liu and Peng Jiang.
Acknowledgements
The study's lead author is Ranjie Xu, a postdoctoral researcher in Jiang's lab. Other Rutgers co-authors include Hyosung Kim, a former post-doc in Jiang's lab; Ronald P. Hart, a professor in the Department of Cell Biology and Neuroscience at Rutgers-New Brunswick; Zhiping P. Pang, an associate professor in the Department of Neuroscience and Cell Biology at Rutgers Robert Wood Johnson Medical School, and Jing-Jing Liu, a former post-doc in Pang's lab. Scientists at the University of Texas Health Science Center, Kent State University, and University of Nebraska Medical Center contributed to the study.
Return to top of page
| |
|
May 29 2019 Fetal Timeline Maternal Timeline News
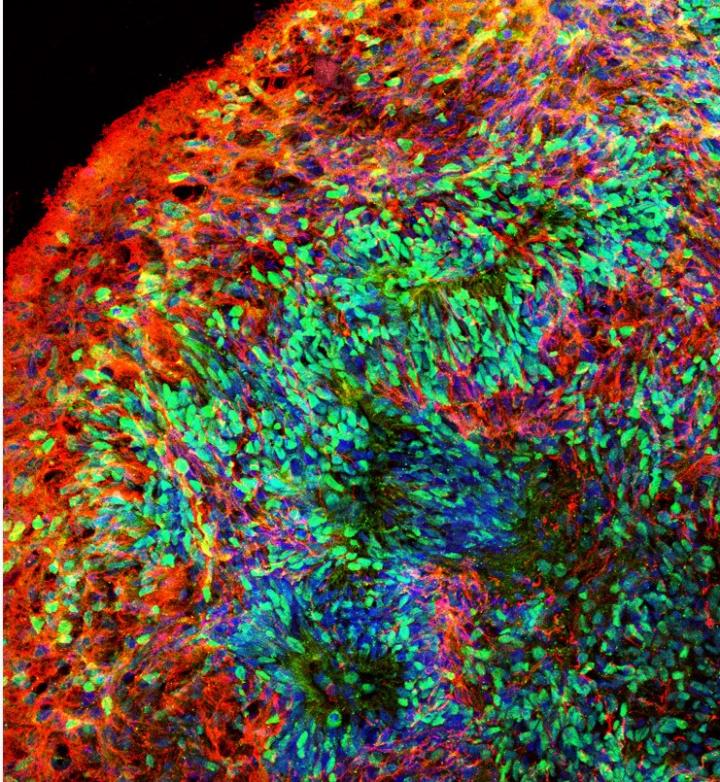 A living 3D "organoid" model of the brain generated from Down syndrome human stem cells. CREDIT Ranjie Xu/Rutgers University-New Brunswick, New Jersey, USA.
|



