|
|
Developmental Biology - Stem Cell Differentation
Landmark Finding Stem Cells Change Fast!!
Landmark study signals shift in thinking about how fast stem cells differentiate...
This paper challenges the longstanding assumption that embryonic stem cells remain quite plastic and malleable during the earliest stages of cell commitment. We show human embryonic stem cells can commit irreversibly to endoderm lineages - liver and pancreas cells for example - very quickly.
David M. Gilbert PhD, Biological Science, Florida State University, Tallahassee, FL 32306, USA
A pioneering new study led by Florida State University (FSU) biologists could fundamentally change our understanding of how embryonic stem cells differentiate into specific cell types.
The research is published in the journal Stem Cell Reports, calls into question decades of scientific consensus about the behavior of embryonic stem cells as they transition to endoderm, a class of cell in animal embryos that gives rise to the digestive and respiratory systems.
David M. Gilbert, the J. Herbert Taylor Distinguished Professor of Molecular Biology in FSU's Department of Biological Science, said the study upends well-established notions of when embryonic stem cells chart their unalterable courses toward a fixed endoderm lineage - in this case, their eventual fate as specific digestive or respiratory cells.
"This paper challenges the longstanding assumption that embryonic stem cells remain quite plastic and malleable during the earliest stages of cell commitment. We show that human embryonic stem cells can commit irreversibly to endoderm lineages - liver and pancreas cells, for example - very quickly."
David M. Gilbert PhD, the J. Herbert Taylor Distinguished Professor of Molecular Biology in FSU's Department of Biological Science.
The findings represent a new chapter in the study of embryonic stem cell differentiation, a field that could be key to helping scientists and clinicians unlock improved therapies for a range of diseases.
Using a sophisticated protocol developed by the San Diego-based regenerative medicine firm ViaCyte, Gilbert and his collaborators exposed a sample of embryonic stem cells to culture conditions engineered to nudge the cells into the definitive endoderm stage, a fast lane to specialized cell development. The team then quickly returned the cells to a bath of treatment factors designed to restore them to an embryonic state.
Based on previous studies, the researchers presumed it would take days in the endoderm culture, or at least a full cell division cycle, for the cells to commit to a developmental track.
"In fact, we found that after only a few hours exposure to the endoderm cocktail - a fraction of a cell division cycle - the cells could be returned to the stem cell cocktail and continue to go through the same series of gene expression changes as the control cells that remained in the endoderm cocktail."
David M. Gilbert PhD
In other words, after a remarkably short soak in the endoderm culture, the cells had committed full bore to a specific cellular program.
"Prior to the experiments reported here, there was no expectation that early stem cell lineage commitment would be so rapid and irreversible," Gilbert and his co-authors wrote in their paper.
This wasn't the only entrenched assumption challenged by the team's study. Scientists long believed the 3D organization of chromosomes in the nucleus to be both exceptionally rigid and closely linked to replication timing - the order in which segments of DNA are copied before cell division. It was thought that the only way to reconfigure that architecture was to crack open a cell's nucleus when its chromosomes were being delivered to its daughter cells.
It turns out those assumptions may have been misguided as well.
"We show that chromosome architecture can be remodeled locally and rapidly without dismantling the entire cell nucleus - akin to changing the scaffolding of a building without tearing it down - which was quite unexpected.
We also show that these changes in chromosome architecture occur dynamically and immediately upon stimulation of stem cells to become endoderm. This finding demonstrates that replication and architecture do not always go hand in hand, they can be what we call 'uncoupled.'"
David M. Gilbert PhD
The researchers' work delinking replication timing from chromosome architecture and showing the ability to surgically remodel that architecture could help refine scientists' understanding of embryonic stem cell behavior. Along with the discovery that stem cell lineage commitment occurs more rapidly and irreversibly than expected, Gilbert said the findings raise critical questions about the basic nature of stem cells and the barriers to turning one cell into another.
If researchers can harness these newly acquired insights, they could begin unraveling the mysteries of how and why stem cells commit to their developmental tracks and why certain cells are especially difficult to reprogram.
That information could inform the creation of new, powerful tools to combat disease and allay human suffering.
"The fact that large changes in genome organization and their temporal order of replication can be remodeled so easily, and that this is correlated with irreversible commitment so quickly in a cell culture system in the laboratory, means that we might be able to use this system to get at the mechanisms that represent irreversible commitment.
We never anticipated that - we expected irreversible commitment to take a lot more work, time and expense."
David M. Gilbert PhD
Highlights
Stable transcriptional reprogramming toward endoderm within one cell cycle
Replication timing can change in the first S phase of a cell fate change
Early discordance between Replication timing and Hi-C compartments
Alignment of replication timing to compartments increases in later cell cycles
Summary
The temporal order of DNA replication is regulated during development and is highly correlated with gene expression, histone modifications and 3D genome architecture. We tracked changes in replication timing, gene expression, and chromatin conformation capture (Hi-C) A/B compartments over the first two cell cycles during differentiation of human embryonic stem cells to definitive endoderm. Remarkably, transcriptional programs were irreversibly reprogrammed within the first cell cycle and were largely but not universally coordinated with replication timing changes. Moreover, changes in A/B compartment and several histone modifications that normally correlate strongly with replication timing showed weak correlation during the early cell cycles of differentiation but showed increased alignment in later differentiation stages and in terminally differentiated cell lines. Thus, epigenetic cell fate transitions during early differentiation can occur despite dynamic and discordant changes in otherwise highly correlated genomic properties.
Authors
Vishnu Dileep, Korey A. Wilson, Claire Marchal, Xiaowen Lyu, Peiyao A. Zhao, Ben Li
, Axel Poulet, Daniel A. Bartlett, Juan Carlos Rivera-Mulia, Zhaohui S. Qin, Allan J. Robins, Thomas C. Schulz, Michael J. Kulik, Rachel Patton McCord, Job Dekker, Stephen Dalton, Victor G. Corces and David M. Gilbert
Acknowlegements
The authors thank R. Didier for assistance with flow cytometry. This work was funded by NIH grants GM085354 and GM083337 to D.M.G. and HG003143 to J.D. A.J.R. and T.C.S. are employees of Viacyte receiving salary and stock options.
Return to top of page.
| |
|
Jun 24 2019 Fetal Timeline Maternal Timeline News
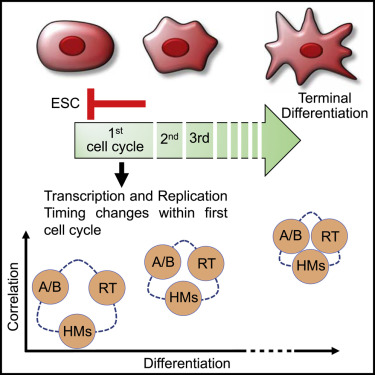 Transcription and replication timing changes take place within the first cell cycle. CREDIT Gilbert labs Florida State University.
|



