|
|
Developmental Biology - Nerves
Schwann Cells Generate Myelin
New discovery could lead to healing nervous system disorders...
Scientists have discovered that a special type of cell called the Schwann cell, is more prolific in generating a protective sheath to cover nerve fibers than previously thought. Schwann cells are any of the cells in the peripheral nervous system that produce the myelin sheath around neuronal axons. Schwann cells are named after German physiologist Theodor Schwann, who discovered them in the 19th century, and are equivalent to a type of neuroglia called oligodendrocytes, which occur in the central nervous system.
Schwann cells differentiate from cells of the neural crest during early embryo development, they are stimulated to proliferate by something on the axon surface. When motor neurons are severed, nerve terminals degenerate. However, degeneration can be followed by regeneration; nerve fibres can regenerate and return to their original target sites. Schwann cells that remain after nerve degeneration apparently determine this return route.
This revelation about other forms of neuropathy [disease or injury] could prove useful in promoting myelin repair in central nervous system disorders such as multiple sclerosis, where damage to myelin slows or blocks electric signals from the brain.
"This totally overturns the textbook definition of the way Schwann cells work,"
Kelly Monk PhD, professor and co-director of the Vollum Institute at Oregon Health & Science University.
The work is published in the journal Nature Communications.
Two types of cells in the body produce myelin: oligodendrocytes in the brain and spinal cord, and Schwann cells in the rest of the body. Until now, scientists thought only oligodendrocytes generated multiple myelin sheaths around axons — those slender nerve cell projections that carry electrical signals between cells.
The new research reveals that Schwann cells also are capable of spreading myelin across multiple axons.
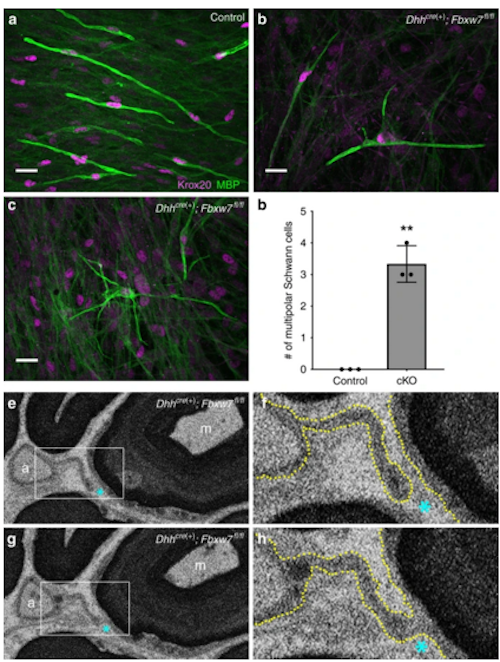
Researchers made the discovery after conducting a genetic screen of zebrafish in the Monk laboratory, discovering some fish had more myelin than expected. Those particular fish carried a mutation in the gene called fbxw7. When they knocked out the fbxw7 gene in mice, the reearchers observered an unexpected characteristic: individual Schwann cells began spreading myelin across many axons.
"This highlights a very plastic potential for these cells," according to Monk.
Discovering how Schwann cells generate myelin at the molecular level, may lead to new gene-therapy techniques to repair damaged myelin in the peripheral nervous system. Disorders such as Charcot-Marie-Tooth disease, a painful inherited form of neuropathy affecting 1 in 2,500 people in the United States alone.
Both Schwann cells and oligodendrocytes arose at the same point in evolutionary history, with the appearance of jaws in the vertebrate lineage. Invertebrates lack myelin, and some, like the modern squid, uses thick axons to quickly transmit signals between neurons. "We could have evolved that way, but our spinal cord would be the diameter of a giant sequoia tree," Monk said.
Vertebrate axons evolved myelin to protect axons and speed up signal transmission. Schwann cells evolved to produce myelin around a single axon in the peripheral nervous system. Oligodendrocytes, in turn, generated myelin along multiple axons within the more confined environment of the brain and spine - the central nervous system.
"The real estate is fundamentally different in the central nervous system than in the peripheral nervous system," adds Monk.
Monk theorizes that Schwann cells evolved a mechanism to repair damaged myelin on a cell by cell basis, as it would have been common for injuries to occur without necessarily killing the entire organism. Those traits would have been passed down and strengthened through generations of evolution. In contrast, remyelination in the central nervous system tended to be an evolutionary dead end as few would have survived a severe whack to the brain or spine.
"There's no selective pressure in repairing myelin damage in the central nervous system, because you're probably going to die," Monk said.
However, the discovery published suggests a new opportunity to heal the brain and spine.
"Targeting the fbxw7 gene - or downstream pathway molecules - could be a powerful way to promote myelin repair in the central nervous system."
Kelly R. Monk PhD, Department of Developmental Biology, Washington University School of Medicine, St. Louis, Missouri, USA; Vollum Institute, Oregon Health & Science University, Portland, Oregon, USA.
Abstract
In the central nervous system (CNS), oligodendrocytes myelinate multiple axons; in the peripheral nervous system (PNS), Schwann cells (SCs) myelinate a single axon. Why are the myelinating potentials of these glia so fundamentally different? Here, we find that loss of Fbxw7, an E3 ubiquitin ligase component, enhances the myelinating potential of SCs. Fbxw7 mutant SCs make thicker myelin sheaths and sometimes appear to myelinate multiple axons in a fashion reminiscent of oligodendrocytes. Several Fbxw7 mutant phenotypes are due to dysregulation of mTOR; however, the remarkable ability of mutant SCs to ensheathe multiple axons is independent of mTOR signaling. This indicates distinct roles for Fbxw7 in SC biology including modes of axon interactions previously thought to fundamentally distinguish myelinating SCs from oligodendrocytes. Our data reveal unexpected plasticity in the myelinating potential of SCs, which may have important implications for our understanding of both PNS and CNS myelination and myelin repair..
Authors
Breanne L. Harty, Fernanda Coelho, Sarah E. Pease-Raissi, Amit Mogha, Sarah D. Ackerman, Amy L. Herbert, Robert W. Gereau IV, Judith P. Golden, David A. Lyons, Jonah R. Chan and Kelly R. Monk.
Acknowledgements
The authors thank the Monk lab (Washington University [WU] and the Vollum Institute at Oregon Health & Science University [OHSU]) for valuable discussions, as well as the Solnica-Krezel and Johnson laboratories (WU) for helpful feedback. The Cavalli Lab (WU), especially Dan Carlin, supplied helpful reagents and useful feedback. We also thank the Nechiporuk lab (OHSU) for helpful input and advice. We are indebted to Laura Feltri, Carmen Melendez-Vasquez, and Steve Scherer for helpful discussions and to Megan Corty and Ben Emery for critical comments on the paper. Electron microscopy was performed at the Multiscale Microscopy Core with technical support from the OHSU Center for Spatial Systems Biomedicine. Specifically, we thank Claudia Lopez, Jessica Riesterer, and Kevin Loftis for the help with imaging and analyzing the 3D SBF-SEM data. This work was supported by NIH/NINDS to B.L.H. (F31 NS094004); NIH/NINDS to S.D.A. (F31 NS087801); NIH/NINDS to A.L.H. (F31 NS096814); NIH/NINDS to R.W.G. (R01 NS042595); NIH/NINDS to J.R.C. (R01NS062796); a Wellcome Senior Research Fellowship (D.A.L.), the Edward J. Mallinckrodt Jr. Foundation (K.R.M.), the Race to Erase MS Foundation (K.R.M.), and K.R.M. is a Harry Weaver Neuroscience Scholar of the National Multiple Sclerosis Society.
Return to top of page.
| |
|
Jul 11 2019 Fetal Timeline Maternal Timeline News
 The gene Fbxw7 orchestrates Schwann Cells (SC) to expand using the mammalian target of rapamycin (mTOR), also known as the mechanistic targets of rapamycin and FK506-binding protein or mTOR-dependent and -independent mechanisms.
Artistic renditions of the phenotypes observed in Fbxw7 mutant SCs (right) as compared with normal SCs (left). Fbxw7 is involved in radial sorting (top), as well as both mature myelinating SCs (middle) and Remak SCs (bottom). A simplified PI3K/mTOR pathway shows that in SCs, Fbxw7 directly inhibits mTOR, and thus regulates multiple aspects of SC biology. Fbxw7 may regulate SC myelinating potential through its control of c-Jun. Steps in the pathway that were not demonstrated directly in this study are shown in gray. Pencil sketches by B.L.H.
|



