|
|
Developmental Biology - Insulin Resistance
Reversing Diabetes
A potential therapeutic strategy for diabetes...
Scientists changed the trajectory of metabolic disease by deactivating an enzyme called dihydroceramide desaturase 1 (DES1). Doing so stopped it from removing the final hydrogens from a fatty lipid called ceramide, effectively lowering the total amount of ceramide in the body.
This finding highlights ceramide's role in metabolic health, pinpointing DES1 as a "druggable" target perhaps useful in new therapies for metabolic disorders such as prediabetes, diabetes and heart disease which affect hundreds of millions of Americans. Scientists at the University at Utah Health and Merck Research Laboratories led the research, published online in Science July 4, 2019.
The research was carried out as a collaboration between investigators at University of Utah Health; Merck Research Laboratories; Baker IDI Heart and Diabetes Institute, University of Brunei Darussalam; and SCIEX.
"We have identified a potential therapeutic strategy that is remarkably effective, and underscores how complex biological systems can be deeply affected by a subtle change in chemistry," says Scott Summers PhD, Chair of the Department of Nutrition and Integrative Physiology and the Diabetes and Metabolism Research Center, at the University of Utah, Salt Lake City. He and co-senior author David E. Kelley MD, recently retired from Merck Research Laboratories, led the research. "Our work shows that ceramides have an influential role in metabolic health," says Summers. "We're thinking of ceramides as the next cholesterol."
This isn't the first time that Summers' group has found lowering ceramides could reverse signs of diabetes and metabolic disease. But, previous techniques caused severe side effects. This time, they made the smallest change possible - shifting the position of two hydrogen atoms - at a precise time blocking the final step of ceramide synthesis.
Scott A. Summers' group genetically engineered mice in which the gene coding for DES1 could be switched off during adulthood - deactivating it from either liver or fat cells.
David E. Kelley MD led a group which injected short hairpin RNA into the adult mouse liver, a method that selectively lowered production of DES1 by destroying it's RNA precursor.
Both approaches were tested first by placing adult mice on a high-fat diet, one with plenty of sugar and six times the fat of a normal rodent diet. The mice gained two times their body weight within three months. Along with obesity came a strain on their metabolic health. They developed insulin resistance and fat accumulated in their livers - two signs of metabolic disease.
Within weeks of lowering ceramides using either technique, there were significant changes. Mice remained obese but their metabolic health improved. Fat cleared from the liver and they responded to both insulin and glucose like a healthy, skinny mouse. In contrast to previous interventions, mice remained healthy during the two-month investigation. However, long-term effects on health are still under investigation.
"Their weight didn't change but the way they handled nutrients did," says Summers. "The mice were fat but they were happy and healthy."
In another example, lowering ceramides in mice before putting them on a high-fat diet, prevented their weight gain and insulin resistance. Lowering ceramides in humans is still unexamined, but there is some evidence they are linked to metabolic disease. Summers points out how clinics already perform ceramide screening tests to gauge an individual's risk for developing heart disease.
Summers and Kelley are now developing drugs to inhibit DES1 with the intention of making new therapeutics.
When the Good Goes Bad
If ceramides cause poor health, why do we have them in the first place? Summers' group addressed the question by measuring how lipids affect metabolism.
Ceramides were found to trigger a number of cell mechanisms promoting storage of fat in cells, which can also impair a cells' ability to use glucose, a type of sugar, for fuel. Evidence of this is activation of the molecular pathway, Akt/PKB, that stops synthesis of sugars from the bloodstream.
Ceramides can also slow turnover of fatty acids partly by causing liver cells to store more fatty acid - and adipose (fatty) tissue to burn less fat.
A shift in how cells use fuel is an advantage to the cell in the short-term, explains Summers. Ceramides have another role, they can stiffen a cell membrane, promoting fat storage — increases production of ceramides. This suggests one benefit of ceramides is protecting the cell. When food is plentiful and cells store lots of fat, higher ceramide levels strengthen the cells' outer membrane, preventing ruptures.
"Serving in this role is usually good but it can, potentially, be bad," explains Trevor Tippetts, a graduate student in Summers' lab. He explains - problems arise in times of chronic overabundance, such as in obesity when persistently high levels of ceramides exist. Continued instability in metabolic equilibrium leads to insulin resistance and fatty liver disease — hinting at ceramide's normal role.
"We think ceramides evolved as a nutritional sensor, to serve as a signal helping the body cope when the amount of fat coming into a cell exceeds its energy need and storage capacity."
Bhagirath Chaurasia PhD
Abstract
Ceramides contribute to the lipotoxicity that underlies diabetes, hepatic steatosis, and heart disease. By genetically engineering mice, we deleted the enzyme dihydroceramide desaturase-1 (DES1) which normally inserts a conserved double bond into the backbone of ceramides and other predominant sphingolipids. Ablation of DES1 from whole animals, or tissue-specific deletion in the liver, and/or adipose tissue resolved hepatic steatosis and insulin resistance in mice caused by leptin deficiency or obesogenic diets. Mechanistic studies revealed new ceramide actions that promoted lipid uptake and storage and impaired glucose utilization, none of which could be recapitulated by (dihydro)ceramides that lacked the critical double bond. These studies suggest that inhibition of DES1 may provide a means of treating hepatic steatosis and metabolic disorders.
Authors
Bhagirath Chaurasia, Trevor S. Tippetts, Rafael Mayoral Monibas, Jinqi Liu, Ying Li, Liping Wang, Joseph L. Wilkerson, C. Rufus Sweeney, Renato Felipe Pereira, Doris Hissako Sumida, J. Alan Maschek, James E. Cox, Vincent Kaddai, Graeme Iain Lancaster, Monowarul Mobin Siddique, Annelise Poss, Mackenzie Pearson, Santhosh Satapati, Heather Zhou, David G. McLaren, Stephen F. Previs, Ying Chen, Ying Qian, Aleksandr Petrov, Margaret Wu, Xiaolan Shen, Jun Yao, Christian N. Nunes, Andrew D. Howard, Liangsu Wang, Mark D. Erion, Jared Rutter, William L. Holland, David E. Kelley, Scott A. Summers.
Acknowledgements
Researchers received support from the National Institutes of Health, American Diabetes Association, Juvenile Diabetes Research Foundation, American Heart Association, and the Margolis Foundation.
Summers and Kelley are shareholders and consultants with Centaurus Therapeutics.
About University of Utah Health
University of Utah Health provides leading-edge and compassionate medicine for a referral area that encompasses 10% of the U.S., including Idaho, Wyoming, Montana and much of Nevada. A hub for health sciences research and education in the region, U of U Health has a $356 million research enterprise and trains the majority of Utah's physicians and more than 1,250 health care providers each year at its Schools of Medicine and Dentistry and Colleges of Nursing, Pharmacy and Health. With more than 20,000 employees, the system includes 12 community clinics and four hospitals. For nine straight years, U of U Health has ranked among the top 10 U.S. academic medical centers in the rigorous Vizient Quality and Accountability Study, including reaching No. 1 in 2010 and 2016.
Return to top of page.
| |
|
Jul 12 2019 Fetal Timeline Maternal Timeline News
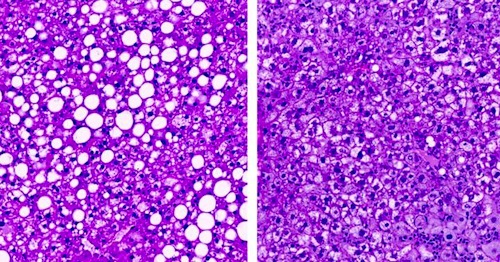 (LEFT) Fatty liver in obese mouse. (RIGHT) Liver in mice in which ceramides were lowered by inactivating an enzyme, dihydroceramide desaturase 1, a target that could be used to develop new drugs against diabetes. CREDIT Trevor Tippetts.
|



