|
|
Developmental Biology - Gene Formation
From Chaos Comes Order
From the tiny testes of flies, insight into where and when some genes are changed...
In the battle of the sexes, males appear to have the innovative edge - from a genetic standpoint. Scientists are finding that testes are more than mere factories for sperm. These organs also serve as hotspots for the emergence of new genes — the raw material for the evolution of species.
Using fruit flies, a research team from Rockefeller University has gained key insight into how nature's attempts at innovation play out during sperm development. In research described August 16 in eLife, they mapped the presence of mutations to DNA at the single-cell level, and the activity of new genes arising from such changes.
"Our work offers an unprecedented perspective on a process that enables living things to adapt and evolve; ultimately contributing to the diversity of life on Earth," says assistant professor Li Zhao, research leader.
High stakes
In recent years, studies in animals from flies to humans have turned up a number of young genes that originated in the testes. These and other discoveries suggest the testes rank among the most productive sites in the body - male or female - for genetic innovation.
This mass production of genetic novelties comes with significant risks, however.
In humans, for example, a father's sperm acquires two to three times more new mutations than do a mother's eggs in the course of normal development, leaving the sperm riddled with genetic mistakes. In some cases, such mistakes may harm his offspring, or even derail the prospect of fatherhood altogether.
In other words, the male stands to lose the one thing that matters in the game of evolution: the opportunity to propagate his gene pool into the next generation.
But whatever the potential downside genetic experimentation has for individual males and their offspring, the dynamics of reproduction nevertheless encourage it. Potential fathers face intense pressure to attract females and fend off competitors. Any advantage, such as brighter plumage or hardier sperm, for example, can make all the difference.
At the molecular level, this pressure drives an abundance of new genes within testes. Scientists think that if these newcomers contribute to males' ability to father healthy offspring, they rapidly acquire a fixed place in the genome and may even go on to contribute elsewhere in the body.
Searching Cell by Cell
Looking a little closer, however, the picture gets blurry. Scientists haven't yet understood the dynamics by which genetic innovation occurs within the precursor cells from which sperm develops.
To find out more, Zhao and researchers in her lab tagged individual cells from fly testes, then identified and decoded the RNA sequences each contained. This approach allowed them to see how the activity of specific genes changed throughout the developmental stages. Within the RNA sequences isolated from stem cells and five intermediary cell types, the researchers examined innovation from two perspectives: that of mutations and that of genes.
Mutations known as substitutions, in which one letter of DNA's code is swapped for another, are most abundant early in the development of sperm, then decrease, researchers found. The sperm cells' DNA repair machinery follows a similar pattern - more active early on, then tapering off - which makes sense, according to Zhao, as the machinery is responsible for fixing errors like these.
Starting from scratch
Within the RNA sequences, Zhao's team hunted for a particular type of young gene - one that arises from scratch rather than through duplication of an existing gene.
For Zhao, these so-called de novo genes, which originate from sequences that originally did not code for protein, are the most interesting new genes from an evolutionary perspective. Her team found no less than 184 de novo genes, drawn from a set they had previously identified.
When examined, the scientists uncovered complex patterns in these de novo genes, with certain genes showing up primarily in certain cell types — but not in others. About 15 percent of these genes appeared early on, including in the stem cell stage - which is surprising, Zhao says, because scientists previously thought that new genes rarely show up at the start of development — a tightly controlled phase. The most active period for de novo genes occurred midstream, however, in the so-called spermatocyte phase of developing sperm.
The scientists are now interested in understanding what purpose, if any, de novo genes serve when they first arise. And although it's possible that some essentially fire at random, making no particular contribution, Zhao suspects in many cases, these new genes play roles in the maturation of sperm cells.
"Precisely what these de novo genes are doing to move development along is an exciting open question."
Li Zhao PhD, Assistant Professor, Laboratory of Evolutionary Genetics and Genomics, The Rockefeller University, New York, United States.
Abstract
The testis is a peculiar tissue in many respects. It shows patterns of rapid gene evolution and provides a hotspot for the origination of genetic novelties such as de novo genes, duplications and mutations. To investigate the expression patterns of genetic novelties across cell types, we performed single-cell RNA-sequencing of adult Drosophila testis. We found that new genes were expressed in various cell types, the patterns of which may be influenced by their mode of origination. In particular, lineage-specific de novo genes are commonly expressed in early spermatocytes, while young duplicated genes are often bimodally expressed. Analysis of germline substitutions suggests that spermatogenesis is a highly reparative process, with the mutational load of germ cells decreasing as spermatogenesis progresses. By elucidating the distribution of genetic novelties across spermatogenesis, this study provides a deeper understanding of how the testis maintains its core reproductive function while being a hotbed of evolutionary innovation.
Authors
Evan Witt, Sigi Benjamin, Nicolas Svetec, Li Zhao Is a corresponding author all of The Rockefeller University, United States.
Acknowledgements
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
The authors thank Connie Zhao and Nneka Nnatubeugo for the help on the single-cell sequencing experiment. We thank Kristofer Davie for the suggestions on single-cell suspension. We thank members of the Zhao laboratory for helpful discussions during the work. We are grateful to Mia Levine, Leslie Vosshall, David Begun, Ziyue Gao, Molly Przeworski, Xiaolan Zhao, and Sohail Tavazoie for critical reading of an earlier version of the manuscript.
Return to top of page.
| |
|
Aug 19 2019 Fetal Timeline Maternal Timeline News
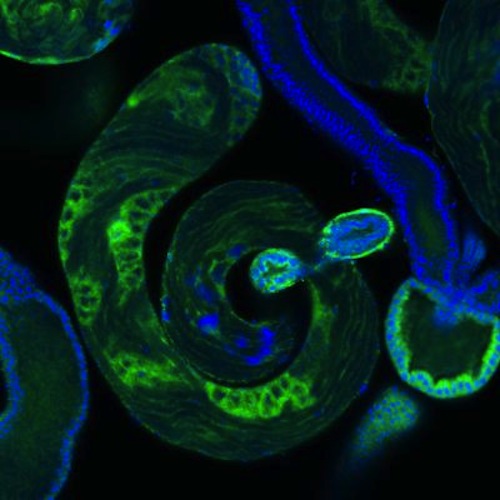 Pictured within a fruit fly testis are developing sperm colored BLUE. Mutations to DNA at the single-cell level, spurs the formation of new genes to arise from these mutations. CREDIT Laboratory of Evolutionary Genetics and Genomics at The Rockefeller University
|



