|
|
Developmental Biology - Over Eating Speeds Up Aging
Cradle to Grave Overeating Linked to Fast Aging
Obesity, beginning in infancy, ruins human health...
Researchers at Baylor College of Medicine have found a new answer to an old question: how can overnutrition during infancy lead to long-lasting health problems such as diabetes? The report, published in the journal Environmental Epigenetics, focuses on the pancreatic Islets of Langerhans, which produce insulin and other hormones.
Islets of mice that were overnourished during the first 21 days after birth (their 'infancy') tended to gain DNA methylation tags, chemical modifications that alter gene expression, while mice that were not overnourished showed similar changes, but much later in life.
"It's been known for several decades that mice overnourished during the suckling period remain overweight and will be prone to disease for their entire lives. Particularly, they have problems regulating blood sugar levels."
Robert A. Waterland PhD, Professor, Pediatrics & Nutrition, USDA/ARS Children's Nutrition Research Center; and of Molecular and Human Genetics, Baylor College of Medicine and Texas Children's Hospital; also corresponding author of this paper.
Looking to shed light on this important topic, Waterland and his colleagues investigated whether early postnatal overnutrition could alter epigenetic development in pancreatic islets that support the pancreas as it further breaks down glucose from the food we eat.
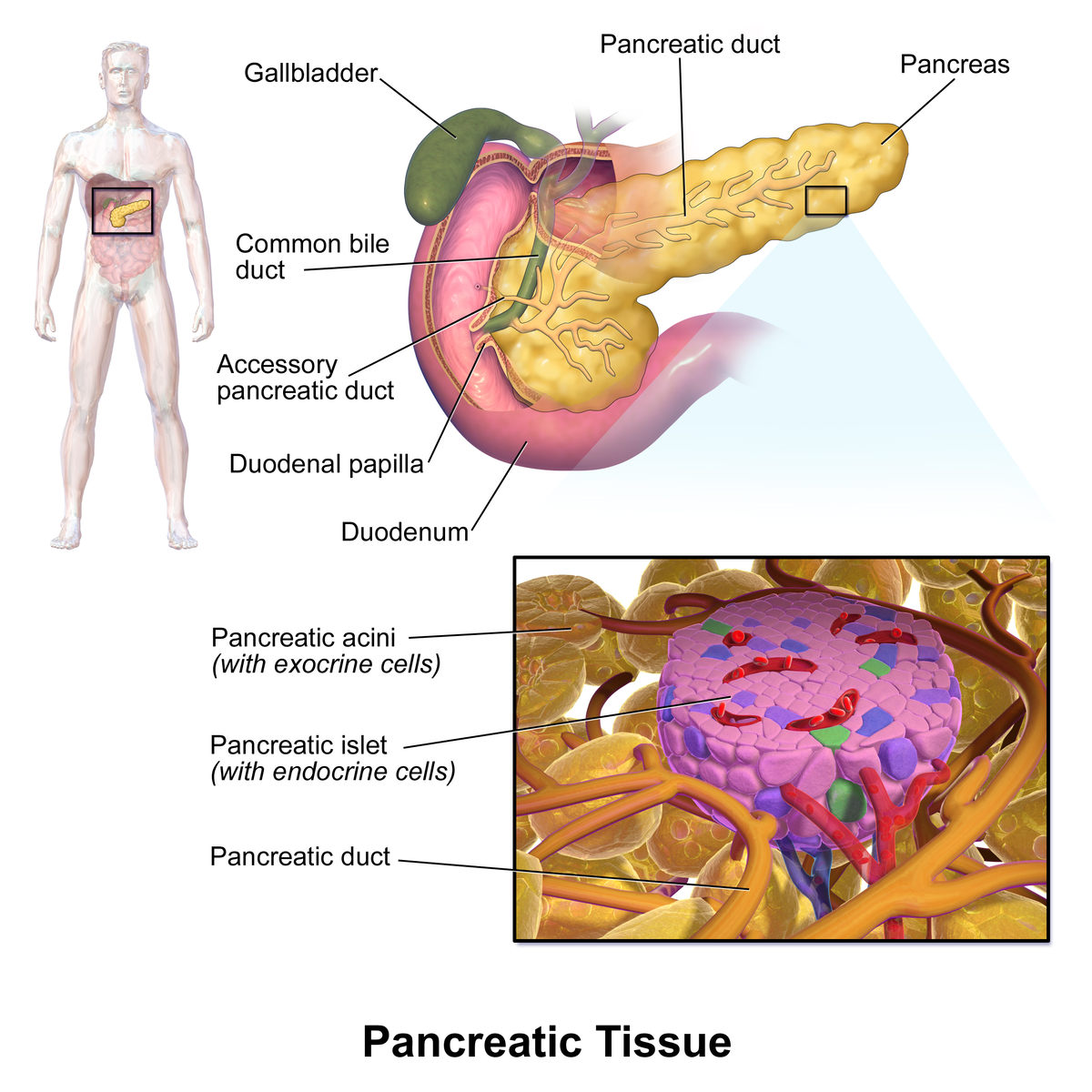
Previous studies also show that patients with Type-2 diabetes have altered DNA methylation - meaning there are added methyl chemical groups in their insulin-producing pancreatic islets. These alterations are linked to islet malfunction and onset of diabetes, but how they occur is still being worked out.
Epigenetics refers to molecular mechanisms that determine which genes will be turned on or off in different cell types. If DNA is the computer hardware, epigenetics is the software that determines what a computer can do. Epigenetics works by adding or removing chemical tags to genes to mark those that should be used and 'turning off' other genes from use.
DNA methylation is one of the most studied chemical tags, critically important in development.
Researchers worked with two groups of mice, one overnourished during infancy and the other not — the latter being the control group. "Reducing litter size during the suckling period provides a natural means to overnourish mouse pups," explains Waterland, also a member of the Dan L Duncan Comprehensive Cancer Center at Baylor. "Normal size litters are about 10 mice, and served as our control group. The overnourished group came from moms whose litters were reduced to only four pups each. These pups get an 'all you can eat buffet' and become overweight by the time of weaning."
But weight was not the only difference between the two groups of pups. Researchers did genome-scale profiling of DNA methylation in islets of overnourished as well as control mice — at 21 days after birth (weaning); and 180 days after birth (considered middle-age for mice).
These results revealed that islets from control mice tended to increase methylation of their DNA as they aged. Compared to control mice, islets of overnourished mice showed these increases in DNA methylation right at weaning. This was unexpected.
"By the age of weaning, islet cells of overnourished mice show an epigenetic profile resembling that of much older mice.
Our interpretation is that postnatal overnutrition causes accelerated epigenetic aging of the islets. As the ability to regulate blood sugar declines with age, this premature epigenetic aging may help explain how overnutrition in infancy increases risk for diabetes later in life."
Robert A. Waterland PhD.
Diabetes is a serious, pervasive health concern worldwide. According to a 2017 report from the Centers for Disease Control and Prevention, 9.3 percent of the U.S. population — about 30 million people — are afflicted, increasing risk of serious health outcomes including premature death, vision loss, heart disease, stroke, kidney failure and amputation of toes, feet, or entire leg - or legs.
"In these days of escalating pediatric overnutrition and obesity, we urgently need to understand the adverse consequences of overnutrition in human infancy. I believe optimizing nutrition [not quantity] during critical periods of development will prove to be an effective approach to prevent adult disease."
Robert A. Waterland.

Abstract
Pancreatic islets of type 2 diabetes patients have altered DNA methylation, contributing to islet dysfunction and the onset of type 2 diabetes. The cause of these epigenetic alterations is largely unknown. We set out to test whether (i) islet DNA methylation would change with aging and (ii) early postnatal overnutrition would persistently alter DNA methylation. We performed genome-scale DNA methylation profiling in islets from postnatally over-nourished (suckled in a small litter) and control male mice at both postnatal day 21 and postnatal day 180. DNA methylation differences were validated using quantitative bisulfite pyrosequencing, and associations with expression were assessed by RT-PCR. We discovered that genomic regions that are hypermethylated in exocrine relative to endocrine pancreas tend to gain methylation in islets during aging (R2 = 0.33, P < 0.0001). These methylation differences were inversely correlated with mRNA expression of genes relevant to ? cell function [including Rab3b (Ras-related protein Rab-3B), Cacnb3 (voltage-dependent L-type calcium channel subunit 3), Atp2a3 (sarcoplasmic/endoplasmic reticulum calcium ATPase 3) and Ins2 (insulin 2)]. Relative to control, small litter islets showed DNA methylation differences directly after weaning and in adulthood, but few of these were present at both ages. Surprisingly, we found substantial overlap of methylated loci caused by aging and small litter feeding, suggesting that the age-associated gain of DNA methylation happened much earlier in small litter islets than control islets. Our results provide the novel insights that aging-associated DNA methylation increases reflect an epigenetic drift toward the exocrine pancreas epigenome, and that early postnatal overnutrition may accelerate this process.
Authors
Ge Li, Tihomira D Petkova, Eleonora Laritsky, Noah Kessler, Maria S Baker, Shaoyu Zhu and Robert A Waterland.
Acknowledgements
The authors thank Adam Gillum (USDA/ARS CNRC) for assistance with figure design.
Funding
This work was supported by grants from NIH/NIDDK (1R01DK081557 and 1R01DK111831) and USDA (CRIS 3092-5-001-059) to R.A.W., and from the Thrasher Research Fund (NR-0136) to G.L.
Contribution Statement
G.L. and T.D.P. performed research and wrote the manuscript. E.L., M.S.B., and S.Z. performed research. N.K. conducted bioinformatic analyses. R.A.W. designed the study and wrote the manuscript. All authors have approved the manuscript for publication. R.A.W. is responsible for the integrity of the work as a whole.
Return to top of page.
| |
|
Sep 4 2019 Fetal Timeline Maternal Timeline News
 Over feeding — beginning in infancy — results in metabolic disease and earlier death.
|





