|
|
Developmental Biology - Puberty Genes
The Switch' from Juvenile to Adult
Is this the "missing link" that determines the move from juvenile to adult?...
Very little is known about how the onset of puberty is controlled in humans, but the discovery of a new gene in the roundworm C. elegans could be the "missing link" that determines when it's time to make this juvenile-to-adult transition.
Two genes, LIN28 and MKRN3, are known to be associated with precocious puberty in humans, where juveniles as young as six may start developing adult features and behaviors.
These genes are found in all animals, including C. elegans, where they control the juvenile-to-adult transition.
Until the new discovery, it was unclear how these two genes are connected. The more obvious signs of the transition of juvenile to adult tend to be external body morphology or shape, including matured genitalia. But, nervous system changes are also happening at the same time. In humans, the maturation of the brain during adolescence is associated with increased vulnerability to a number of neuropsychiatric disorders. So, a better understanding of all of these processes is important in understanding mental health and basic neurobiology.
Two new studies are out from the labs of Douglas Portman PhD, at the University of Rochester Medical Center and David Fitch at New York University, and published in (1) Developmental Cell and (2) eLife.
They identify a new developmental timing mechanism involving a long non-coding RNA in the microscopic roundworm C. elegans. Their research reveals a surprising new molecular mechanism controls the timing of sex-specific changes in body shape as well as the maturation of neural circuits — and changes in behavior.
C. elegans has long been used by researchers to understand fundamental mechanisms in biology. Many of the discoveries made using these worms apply throughout the animal kingdom, leading to a broader understanding of human biology. In fact, three Nobel Prizes in medicine and chemistry have been awarded for discoveries involving C. elegans.
Researchers have identified a new gene that, when disrupted, delays transition from the juvenile to adult stage. Surprisingly this gene, lep-5, does not act like a protein as do most genes. Instead, it functions as a long non-coding RNA (lncRNA), a recently discovered class of genes whose functions largely remain mysterious.
The team observed that this lncRNA is important in promoting the juvenile-to-adult transition by directly interacting with LIN-28 and LEP-2, a C. elegans gene similar to MKRN3. Because human versions of LEP-2 and LIN-28 are both involved in the timing of puberty, this new finding suggests a yet-to-be-discovered lncRNA might be essential to this process in humans as well.
In the roundworm nervous system, some neural circuits undergo a functional transition in males as they become sexually mature adults. These circuits are critical for generating adult-specific behavior important for reproductive success. The male tail also changes in shape to better enable mating behavior. Researchers found this same neural pathway controls both functional maturation of these circuits as well as shape of the tail.
Roundworms carrying mutations in lep-5 become physically mature adults, but their nervous system remains arrested in their juvenile stage — and their tails retain a juvenile form.
Regarding changes in behavior, this pathway regulates timing by acting in the nervous system itself — not in tissue that sends timing signals to the nervous system. Moreover, individual neurons manage their own developmental clocks.
A timed "pulse" of lep-5 activity during an animal's juvenile stage causes LIN-28 to become inactive — allowing transition into adulthood to proceed.
(1) Developmental Cell: "The Long Non-Coding RNA lep-5 Promotes the Juvenile-to-Adult Transition by Destabilizing LIN-28"
Highlights (1)
• lep-5 acts in the heterochronic pathway to promote the larval-to-adult transition
• lep-5 is a ~600 nt, highly structured lncRNA that is conserved across Caenorhabditis
• Like the Makorin LEP-2, lep-5 promotes the degradation of LIN-28 protein
• lep-5 may act as a scaffold to bring LEP-2 into close proximity with LIN-28
Summary
Biological roles for most long non-coding RNAs (lncRNAs) remain mysterious. Here, using forward genetics, we identify lep-5, a lncRNA acting in the C. elegans heterochronic (developmental timing) pathway. Loss of lep-5 delays hypodermal maturation and male tail tip morphogenesis (TTM), hallmarks of the juvenile-to-adult transition. We find that lep-5 is a ~600 nt cytoplasmic RNA that is conserved across Caenorhabditis and possesses three essential secondary structure motifs but no essential open reading frames. lep-5 expression is temporally controlled, peaking prior to TTM onset. Like the Makorin LEP-2, lep-5 facilitates the degradation of LIN-28, a conserved miRNA regulator specifying the juvenile state. Both LIN-28 and LEP-2 associate with lep-5 in vivo, suggesting that lep-5 directly regulates LIN-28 stability and may function as an RNA scaffold. These studies identify a key biological role for a lncRNA: by regulating protein stability, it provides a temporal cue to facilitate the juvenile-to-adult transition.
Authors
Karin C. Kiontke, R. Antonio Herrera, Edward Vuong, Jintao Luo, Erich M. Schwarz, David H.A. Fitch and Douglas S. Portman.
Acknowledgements
This research was supported by the National Institute of General Medical Sciences and National Science Foundation grants to Portman and Fitch.
(2) eLife: "The Makorin lep-2 and the lncRNA lep-5 regulate lin-28 to schedule sexual maturation of the C. elegans nervous system"
Abstract
Sexual maturation must occur on a controlled developmental schedule. In mammals, Makorin3 (MKRN3) and the miRNA regulators LIN28A/B are key regulators of this process, but how they act is unclear. In C. elegans, sexual maturation of the nervous system includes the functional remodeling of postmitotic neurons and the onset of adult-specific behaviors. Here, we find that the lin-28–let-7 axis (the ‘heterochronic pathway’) determines the timing of these events. Upstream of lin-28, the Makorin lep-2 and the lncRNA lep-5 regulate maturation cell-autonomously, indicating that distributed clocks, not a central timer, coordinate sexual differentiation of the C. elegans nervous system. Overexpression of human MKRN3 delays aspects of C. elegans sexual maturation, suggesting the conservation of Makorin function. These studies reveal roles for a Makorin and a lncRNA in timing of sexual differentiation; moreover, they demonstrate deep conservation of the lin-28–let-7 system in controlling the functional maturation of the nervous system.
Authors
Hannah Lawson, Edward Vuong, Renee M Miller, Karin Kiontke, David HA Fitch and Douglas S Portman.
Return to top of page.
|
|
Sep 20 2019 Fetal Timeline Maternal Timeline News
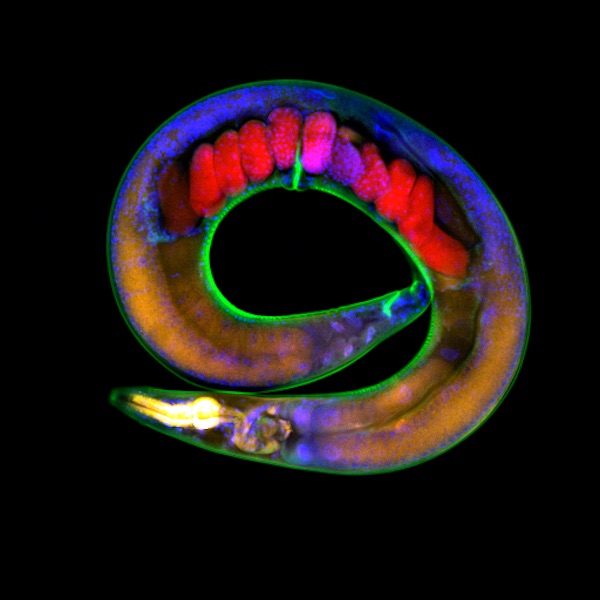 C. elegans worms have a 21-day lifespan with reproductive ability occuring the second and third day of their lives. Soon after, worms begin a steady decline into old age. About day fifteen, most are "old."
|
|



