|
|
Developmental Biology - Synthetic Embryo
New Way to Study Early Development & Pregnancy
Salk synthetic embryo could open new understanding of early development, and applications for human health...
Although graduating from school, a first job and marriage can be important events in life, some of the most significant events happen far earlier — in the first few days after a sperm fertilizes an egg and the cell begins to divide.
The way the first 100 cells (collectively called a blastocyst) organize themselves, has profound implications for whether a pregnancy is successful, for how organs form and potentially even for diseases later in life, such as Alzheimer's. However, scientists have not had a good way to model how a blastocyst is formed, until now.
For the first time, researchers at the Salk Institute and the University of Texas Southwestern Medical Center have created mouse blastocyst-like structures, or "blastoids," from a single cultured cell, circumventing the need for natural embryos.
As they reported October 17, 2019, in the journal Cell, these cultured blastoids have the same structure as natural blastocysts and can even implant in the uterus — and could help advance research into development as well as inform issues around pregnancy, infertility, or health problems later in the offspring's life.
"These studies will help us to better understand the very beginnings of life; how early on in life a single cell can give rise to millions of cells and how they are assembled in space and time to give rise to a fully developed organism. Importantly, this work avoids the use of natural embryos and is scalable," says Juan Carlos Izpisua Belmonte, a professor in Salk's Gene Expression Laboratory.
Natural blastocysts, which can become an embryo once they implant in a uterus, have proven difficult to study. The problem is that animal models, such as mice, only produce these structures in small numbers, and scientists cannot easily test the effects of malnutrition or exposure to toxins or a variety of genetic mutations on development at a level sufficient for study.
"We are optimistic that this work will enable important research into early developmental defects," says Assistant Professor Jun Wu of UT Southwestern, who co-led the study.
The Salk and UT Southwestern teams developed the blastoids using embryonic and, more importantly, adult mouse cells. The adult cells were put into a chemical solution that prompted them to turn into induced pluripotent stem cells, or iPSCs, which can turn into almost any kind of tissue in the body. To encourage iPS cells to form blastoids, researchers put them in small groups in a special culture medium where they soon formed connections with each other. This was exactly what the researchers hoped to see — cells were beginning to form structures similar to the developmental stage before a fertilized egg becomes a blastocyst.
Over time, the connected cells started forming a ball with an inner and outer layer. The cells facing inward accumulated proteins that made them distinct from the outside cells. The cells facing outward also began activating a protein called YAP, which entered the cell nucleus and began the process of inducing expression of proteins to build what could eventually become a placenta.
"The formation of blastoids mimics the natural developmental process," says Ronghui Li, co-first author of the study and a postdoctoral fellow in the Izpisua Belmonte lab.
Blastoids contain the same three primordial cell types found in natural blastocysts — from which all cells of an adult organism come. They are also a similar size as natural blastocysts and show a similar gene signature. Further experiments indicate blastoids could further develop into structures resembling early post-implantation embryos.
"I think this kind of resource is going to be very powerful for studying early development in mammals," says Cuiqing Zhong, co-first author of the study and a postdoctoral fellow in the Izpisua Belmonte lab.
The team next plans to use gene-editing tools to understand how genetic changes in blastoids affect the three different cell types. The blastoids also provide a new model for testing drugs and chemicals for future therapies.
Blastoids cannot develop into functional embryos - instead, the cells grow into disorganized tissue. But the scientists believe the blastoids may reveal details on later stages of embryonic development.
"With further optimization, this technology might lead to the generation of fully functional blastoids able to develop up to the stages when different organ primordia are formed and thus be the seeds for organoids that could be used as invaluable sources for organ transplantation," explains Belmonte.
"The noted physicist Richard Feynman once said, 'What I cannot create, I do not understand.' How life starts from a fertilized egg still remains a mystery, and our blastoid approach will help researchers gain novel insights into this process," added Wu.
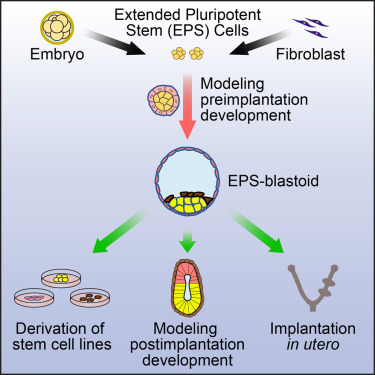
Highlights
• A method that enables the generation of blastocyst-like structures from EPS cells
• EPS-blastoids resemble blastocysts in morphology and cell-lineage allocation
• EPS-blastoid formation recapitulates early developmental events in vitro
• EPS-blastoids are able to implant in utero
Summary
A single mouse blastomere from an embryo until the 8-cell stage can generate an entire blastocyst. Whether laboratory-cultured cells retain a similar generative capacity remains unknown. Starting from a single stem cell type, extended pluripotent stem (EPS) cells, we established a 3D differentiation system that enabled the generation of blastocyst-like structures (EPS-blastoids) through lineage segregation and self-organization. EPS-blastoids resembled blastocysts in morphology and cell-lineage allocation and recapitulated key morphogenetic events during preimplantation and early postimplantation development in vitro. Upon transfer, some EPS-blastoids underwent implantation, induced decidualization, and generated live, albeit disorganized, tissues in utero. Single-cell and bulk RNA-sequencing analysis revealed that EPS-blastoids contained all three blastocyst cell lineages and shared transcriptional similarity with natural blastocysts. We also provide proof of concept that EPS-blastoids can be generated from adult cells via cellular reprogramming. EPS-blastoids provide a unique platform for studying early embryogenesis and pave the way to creating viable synthetic embryos by using cultured cells.
Authors
Ronghui Li, Cuiqing Zhong, Yang Yu, Haisong Liu, Masahiro Sakurai, Leqian Yu, Zheying Min, Lei Shi, Yulei Wei, Yuta Takahashi, Hsin-Kai Liao, Jie Qiao, Hongkui Deng, Estrella Nuñez-Delicado, Concepcion Rodriguez Esteban, Jun Wu, Juan Carlos Izpisua Belmonte.
Acknowledgments
This work was funded by the Larry L. Hillblom Foundation, the Paul F. Glenn Foundation, the National Key R&D Program of China (2016YFC1000601), the G. Harold and Leila Y. Mathers Charitable Foundation, the Moxie Foundation, the Leona M. and Harry B. Helmsley Charitable Trust (2012-PG-MED002), the Hewitt Foundation, the National Institutes of Health (5 DP1 DK113616) and Universidad Católica San Antonio de Murcia.
About the Salk Institute for Biological Studies:
Every cure has a starting point. The Salk Institute embodies Jonas Salk's mission to dare to make dreams into reality. Its internationally renowned and award-winning scientists explore the very foundations of life, seeking new understandings in neuroscience, genetics, immunology, plant biology and more. The Institute is an independent nonprofit organization and architectural landmark: small by choice, intimate by nature and fearless in the face of any challenge. Be it cancer or Alzheimer's, aging or diabetes, Salk is where cures begin. Learn more at: salk.edu.
Return to top of page.
| |
|
Oct 18 2019 Fetal Timeline Maternal Timeline News
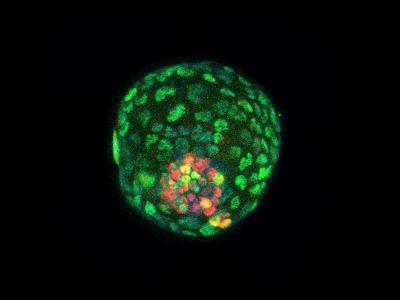
Pictured is a blastocyst-like structure (BLASTOID) from cultured mouse cells immunofluorescently stained. The trophectoderm marker CDX2 (GREEN and the inner cell mass marker SOX2 (RED). Trophoectoderm is the term for the outer cells of the blastoid. CREDIT Salk Institute, Waitt Advanced Biophotonics Core Facility
|




