|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
Developmental Biology - Mitochondrial Disease ANT Protein Mutations Fail to Supress Mitochondria Abstract Mitochondrial homeostasis vitally depends on mitophagy, the programmed degradation of mitochondria. The roster of proteins known to participate in mitophagy remains small. We devised here a multidimensional CRISPR/Cas9 genetic screen, using multiple mitophagy reporter systems and pro-mitophagy triggers, and uncover numerous new components of Parkin-dependent mitophagy1. Unexpectedly, we identify the adenine nucleotide translocator (ANT) complex as required for mitophagy in multiple cell types. While pharmacological inhibition of ANT-mediated ADP/ATP exchange promotes mitophagy, genetic ablation of ANT paradoxically suppresses mitophagy. Importantly, ANT promotes mitophagy independently of its nucleotide translocase catalytic activity. Instead, the ANT complex is required for inhibition of the presequence translocase TIM23, leading to PINK1 stabilization, in response to bioenergetic collapse. ANT modulates TIM23 indirectly via interaction with TIM44, known to regulate peptide import through TIM232. Mice lacking ANT1 reveal blunted mitophagy and consequent profound accumulation of aberrant mitochondria. Disease-causing human mutations in ANT1 abrogate binding to TIM44 and TIM23 and inhibit mitophagy. Together, these data identify a novel and essential function for ANT as a fundamental mediator of mitophagy in health and disease. Authors Atsushi Hoshino, Wei-jia Wang, Shogo Wada, Chris McDermott-Roe, Chantell S. Evans, Bridget Gosis, Michael P. Morley, Komal S. Rathi, Jian Li, Kristina Li, Steven Yang, Meagen J. McMannus, Caitlyn Bowman, Prasanth Potluri, Michael Levin, Scott Damrauer, Douglas C. Wallace, Erika L. F. Holzbaur and Zoltan Arany. Acknowledgments This research was supported by HHMI, Project ALS, HSCI, Target ALS and the NINDS grant NIH5R01NS089742 to K.E. J.R.K. is the Project ALS Tom Kirchhoff Family Postdoctoral Fellow. B.N.D.-D. was supported by the Milton Safenowitz postdoctoral fellowship from the ALS Association. A. Burberry was supported by the US National Institutes of Health (1K99AG057808–01A1). D.A.M. was funded by the MGH training grant (5T32CA009216) and is grateful for the assistance of the Massachusetts ADRC neuropathology core in preparing tissue samples. B.J.W. is a New York Stem Cell Foundation – Robertson Investigator. We thank D. Cleveland for the generous gift of TDP-43 (FL9) antibody. The work was supported, in part, by grants from the National Institutes of Health (HL094499, R37 NS060698, (NS021328, OD010944, MH108592, MH110185 and DK107667), U.S. Department of Defense (W81XWH-16-1-0401) and the established investigator award from the American Heart Association. Penn Medicine is one of the world's leading academic medical centers, dedicated to the related missions of medical education, biomedical research, and excellence in patient care. Penn Medicine consists of the Raymond and Ruth Perelman School of Medicine at the University of Pennsylvania (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System, which together form a $7.8 billion enterprise. The Perelman School of Medicine has been ranked among the top medical schools in the United States for more than 20 years, according to U.S. News & World Report's survey of research-oriented medical schools. The School is consistently among the nation's top recipients of funding from the National Institutes of Health, with $425 million awarded in the 2018 fiscal year. The authors thank the staff of the Penn Medicine Biobank including J. Weaver, D. Birtwell, H. Williams, P. Baumann, and M. Risman, as well as the Regeneron Genetics Center (RGC). The Penn Medicine Biobank was funded by a gift from the Smilow family and by the Penn Cardiovascular Institute and the Perelman School of Medicine. A.H. was supported by the Uehara Memorial Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research and JSPS Overseas Research Fellowships. S.W. was supported by a fellowship from the ADA (1-16-PDF-117) and Toyobo Biotechnology Foundation. C.S.E. was supported by a Hanna H. Gray Fellowship from the Howard Hughes Medical Institute. E.L.F.H. was supported by the NIH NINDS (R37 NS060698). D.C.W. was supported by the NIH (NS021328, OD010944, MH108592, MH110185) and DOD (W81XWH-16-1-0401) Z.A. was supported by the NIH (HL094499, DK107667) and the AHA (Established Investigator Award). Return to top of page. | Oct 24 2019 Fetal Timeline Maternal Timeline News 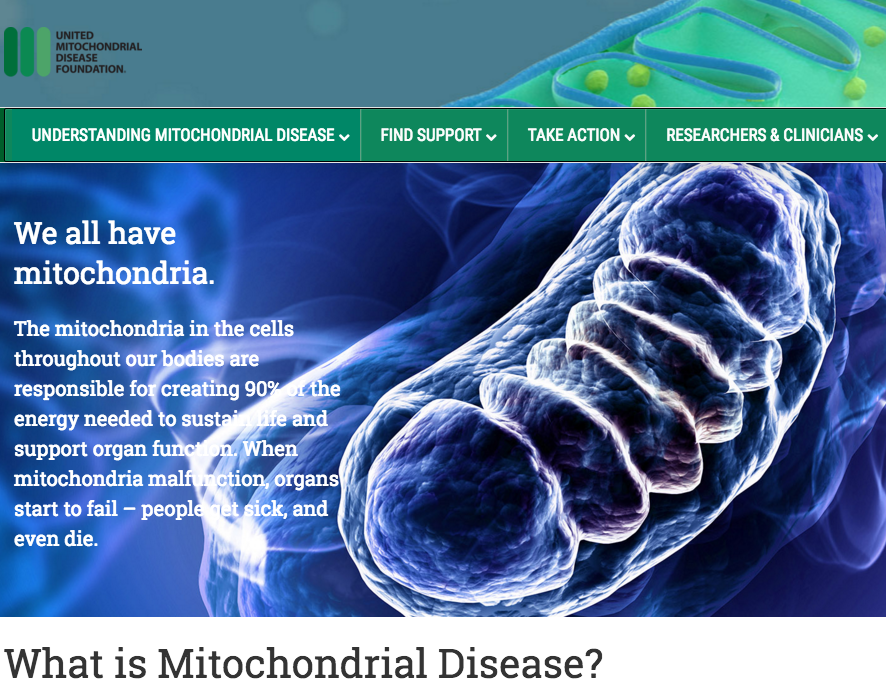 Mitochondria are in cells throughout our bodies. They are responsible for 90% of the energy needed to sustain life and support organ function. When mitochondria malfunction, organs start to fail. CREDIT United Mitochondrial Diesase Foundation.
|
||||||||||||||||||||||||||||

