|
|
Developmental Biology - Cell Fate
Waves of Signals Determine A Cell's Fate
Rice University shows cell-signals distinguish tissue types in embryo...
Timing is everything for young cells waiting to determine their identities.
Research by Rice University bioscientist Aryeh Warmflash and graduate student Sapna Chhabra, show how homogenous colonies of human embryo stem cells use molecular waves to signal from cell to cell and trigger each to differentiate.
Once prompted, the cells begin to organize into the three germ layers - the endoderm, mesoderm and ectoderm - that ultimately become an embryo.
This discovery counters explanations dating back to 1952 research by British mathematician Alan Turing, who argued signaling gradients could self-organize through a mechanism now known as a Turing instability. Such a process would theoretically allow a stable gradient of molecules to deliver signals of different strengths to each cell.
Warmflash, Chhabra and their colleagues show such gradients do not exist in stem-cell colonies and that the process is far more dynamic than previously appreciated.
They observed confined stem-cell colonies and then used mathematical models to determine that such a mechanism could not explain the signaling patterns and waves they saw. Patterns that then trigger differentiation into germ cell layers that become organs, bone, skin and blood.
Their work, published in PLOS Biology, investigates dynamic interactions between the BMP, Wnt and NODAL signaling pathways as part of a long-term study decoding how a single fertilized cell becomes a human being. To closely observe the signalling process, the scientists use specially patterned plates which force stem cells to grow into tiny circular colonies.
Researchers can then observe, measure and perturb the colonies as they progress through the very earliest stages, forming patterns of differentiated cells but never progressing to the point of becoming an embryo.
In the current study, researchers applied BMP (bone morphogenetic protein) to these colonies. Now signals transmitted through this pathway caused cells to start and maintain a wave of cell-to-cell Wnt signals, which travel from the perimeter toward the center of each colony.
Wnt, in turn, initiates a wave of NODAL signals that move independently toward the center. By measuring this cascade, researchers show that:
• The duration of BMP signaling determines the position of the mesoderm or middle layer in development of an early embryo
• While Wnt and NODAL signals increase (upregulate) further mesoderm differentiation.
They also report that interactions between these signaling pathways determines where the mesoderm ring starts and stops.
"We knew the important chemical signals but, until now, no one had observed the activity of these signals in space and time. By focusing on the mesoderm, we showed that differentiation isn't dependent on a particular level of any of the chemical signals a cell uses."
"Now, we know that signaling starts at the colony edge and moves in, and that the position of red (stained mesoderm) cells correlates with where Wnt activity peaks. There's only a certain time period in which they can react."
Sapna Chhabra PhD, Systems, Synthetic and Physical Biology, Rice University, Houston, Texas, United States of America and lead author.
Researchers also observed that cells migrate a little themselves. But, not nearly as fast as the fate altering signals they pass along. Aryeh Warmflash explains:
"These signals are continuously moving, filling in the whole colony. But, depending on when a signal gets to a particular cell, that cell either will or won't respond. By the time the wave reaches cells at the center, those cells have already become ectoderm.
"The controversy in the field over whether these cells represent placenta-like cells or not, is because the decision should have happened before our whole model even begins. But, our new model has allowed us to make the first comparison between these two states. The bottom line is these cells are as good a model for the human placenta as stem cells are for the human embryo. It's not perfect, but it's a reasonably good model."
Aryeh Warmflash PhD,
Project Administrator, Department of Bioengineering, Rice University, Houston, Texas, United States of America.
Abstract
During gastrulation, the pluripotent epiblast self-organizes into the 3 germ layers—endoderm, mesoderm and ectoderm, which eventually form the entire embryo. Decades of research in the mouse embryo have revealed that a signaling cascade involving the Bone Morphogenic Protein (BMP), WNT, and NODAL pathways is necessary for gastrulation. In vivo, WNT and NODAL ligands are expressed near the site of gastrulation in the posterior of the embryo, and knockout of these ligands leads to a failure to gastrulate. These data have led to the prevailing view that a signaling gradient in WNT and NODAL underlies patterning during gastrulation; however, the activities of these pathways in space and time have never been directly observed. In this study, we quantify BMP, WNT, and NODAL signaling dynamics in an in vitro model of human gastrulation. Our data suggest that BMP signaling initiates waves of WNT and NODAL signaling activity that move toward the colony center at a constant rate. Using a simple mathematical model, we show that this wave-like behavior is inconsistent with a reaction-diffusion–based Turing system, indicating that there is no stable signaling gradient of WNT/NODAL. Instead, the final signaling state is homogeneous, and spatial differences arise only from boundary effects. We further show that the durations of WNT and NODAL signaling control mesoderm differentiation, while the duration of BMP signaling controls differentiation of CDX2-positive extra-embryonic cells. The identity of these extra-embryonic cells has been controversial, and we use RNA sequencing (RNA-seq) to obtain their transcriptomes and show that they closely resemble human trophoblast cells in vivo. The domain of BMP signaling is identical to the domain of differentiation of these trophoblast-like cells; however, neither WNT nor NODAL forms a spatial pattern that maps directly to the mesodermal region, suggesting that mesoderm differentiation is controlled dynamically by the combinatorial effect of multiple signals. We synthesize our data into a mathematical model that accurately recapitulates signaling dynamics and predicts cell fate patterning upon chemical and physical perturbations. Taken together, our study shows that the dynamics of signaling events in the BMP, WNT, and NODAL cascade in the absence of a stable signaling gradient control fate patterning of human gastruloids.
Authors
Sapna Chhabra, Lizhong Liu, Ryan Goh, Xiangyu Kong and Aryeh Warmflash
Acknowledgments
The authors thank Idse Heemskerk for helpful discussions on the project; Eric Siggia for early discussions and versions of the simulation code; Barrett Harvey for providing the NODAL mAb11 antibody; Elena Camacho Aguilar, Eleni Anastasia Rizou, and Joseph Massey for careful reading of the manuscript; Cecilia Guerra for technical assistance with experiments; and all the members of Warmflash lab for helpful feedback.
Rice, the Cancer Prevention and Research Institute of Texas, the Simons Foundation and the National Science Foundation funded the research.
Return to top of page.
| |
|
Oct 31 2019 Fetal Timeline Maternal Timeline News
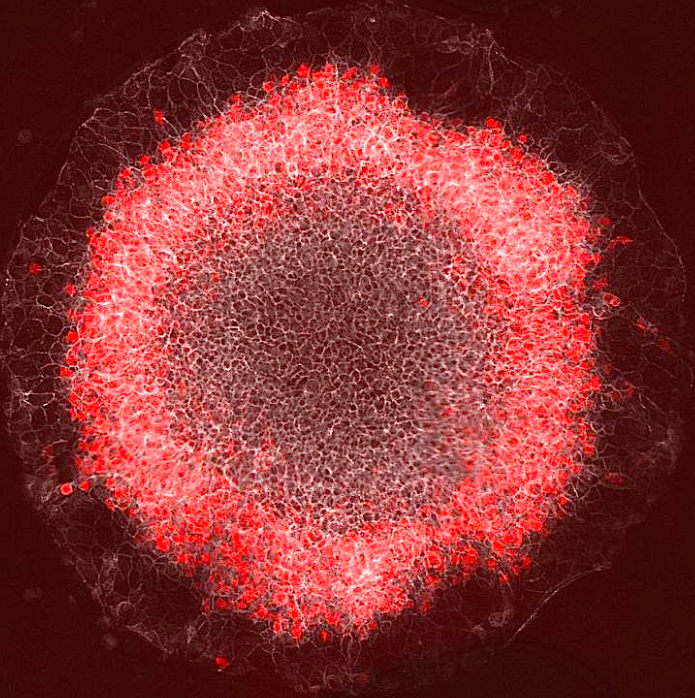
A ring of red cells representing the mesoderm germ layer appear in a stem-cell gastrulation model developed by a Rice University lab. Embryonic stem cells begin to self-organize when they sense interacting waves of molecular signals that help them start and stop - differentiating into patterns ultimately guiding cells toward becoming skin, bone, nerve, organs and blood. CREDIT Courtesy of the Warmflash lab).
|



