|
|
Developmental Biology - Alexander Disease
Alexander Disease Gene Editing Discovery
Scientists identify underlying molecular mechanisms of Alexander disease...
University of North Carolina (UNC) School of Medicine researchers have used human induced pluripotent stem cells and CRISPR/Cas9 gene editing to make discoveries about the molecular structure of Alexander disease, a rare neurodegenerative condition.
Scientists knew genetic mutations lead to the production of GFAP - a defective protein - that causes Alexander disease (AxD). This debilitating neurodegenerative disease can present in infancy, adolescence, even adulthood. Many people with the condition die within the first few years of diagnosis, but some survive for decades.
Now, UNC School of Medicine researchers are learning about the differences in the underlying biology of patients with mild to severe forms of AxD. Led by Natasha Snider PhD, UNC assistant professor of cell biology, an international group of scientists has discovered that the mutant form of GFAP undergoes different chemical modifications, depending on the timing of symptom onset.
Published in the online journal eLife, this research marks the first time scientists have been able to model very specific chemical changes in GFAP that occur inside the AxD brain. Using an in vitro system derived from AxD patient cells, Snider and her colleagues probed the details of how GFAP misfolds; and, how its accumulation alters cellular mechanics leading to disease progression and death.
"We are now investigating the enzymes responsible for key reactions inside brain cells that lead to AxD. We believe our research may open the door to new drug development opportunities - and ultimately new kinds of therapies for people with this terrible disease."
Natasha T Snider PhD,
Department of Cell Biology and Physiology, University of North Carolina at Chapel Hill, Chapel Hill, United States.
AxD is a leukodystrophy, a rare group of disorders of the nervous system. It involves the destruction of myelin, the fatty sheath insulating long connective nerve cells promoting electrical impulses. But, myelin deteriorates in people with AxD and other leukodystrophies.
Most cases of Alexander disease occur during infancy and involve myelin destruction. Babies with AxD have enlarged brains, and they experience seizures, stiffness in the arms and legs, and developmental delay.
Sometimes, though, symptoms do not occur until late in childhood or even appear in adulthood. In the absence of leukodystrophy, symptoms include speech abnormalities, swallowing difficulties, seizures, and poor coordination.
Over time, abnormal protein deposits containing GFAP (known as Rosenthal fibers) accumulate in specialized cells called astrocytes that support and nourish other cells in the brain and spinal cord.
Since 2011, Snider has been studying the mechanisms of GFAP accumulation in the hope of finding an existing drug or compound to help AxD patients. GFAP forms intermediate filaments - structures that shape the 'skeleton' of astrocytes.
Toxic accumulation of GFAP leads to astrocyte dysfunction, which harms surrounding neuronal and non-neuronal cells in AxD patients. Problems of GFAP accumulation in astrocytes can also be found in other diseases, such as giant axonal neuropathy and astrocytoma tumors.
For the eLife study, Snider and colleagues combined mass spectrometry-based proteomic analysis of AxD and non-AxD human brain tissue with induced pluripotent stem cells and CRISPR/Cas9 gene editing technology. This connection of relevant disease phenotypes to the underlying cell biology illuminated key mechanisms involved in GFAP misfolding, revealing new markers of disease severity.
For the first time, researchers made a clear molecular distinction between AxD children who die young and those with AxD who live for several decades.
Using their cell line model created in the lab, team members observed specific types of GFAP aggregates sequestered outside the misshapen membranes of cell nuclei.
"This phenomenon had been previously observed in AxD patient astrocytes. But, ours is the first lab demonstration of this in a model cell line probing GFAP accumulation - and how it affects other organelles to cause disease."
Natasha T. Snider PhD
The findings also relate to published literature on other debilitating and fatal human diseases associated with defects in intermediate filament proteins with similar functions to GFAP.
The next step is to see if these molecular markers of GFAP aggregation lead to the creation of new treatments that help Alexander Disease patients.
Abstract
Alexander Disease (AxD) is a fatal neurodegenerative disorder caused by mutations in glial fibrillary acidic protein (GFAP), which supports the structural integrity of astrocytes. Over 70 GFAP missense mutations cause AxD, but the mechanism linking different mutations to disease-relevant phenotypes remains unknown. We used AxD patient brain tissue and induced pluripotent stem cell (iPSC)-derived astrocytes to investigate the hypothesis that AxD-causing mutations perturb key post-translational modifications (PTMs) on GFAP. Our findings reveal selective phosphorylation of GFAP-Ser13 in patients who died young, independently of the mutation they carried. AxD iPSC-astrocytes accumulated pSer13-GFAP in cytoplasmic aggregates within deep nuclear invaginations, resembling the hallmark Rosenthal fibers observed in vivo. Ser13 phosphorylation facilitated GFAP aggregation and was associated with increased GFAP proteolysis by caspase-6. Furthermore, caspase-6 was selectively expressed in young AxD patients, and correlated with the presence of cleaved GFAP. We reveal a novel PTM signature linking different GFAP mutations in infantile AxD.
Authors
Rachel A Battaglia, Adriana S Beltran, Samed Delic, Raluca Dumitru, Jasmine A Robinson, Parijat Kabiraj, Laura E Herring, Victoria J Madden, Namritha Ravinder, Erik Willems, Rhonda A Newman, Roy Andrew Quinlan, James E Goldman, Ming-Der Perng, Masaki Inagaki, Natasha T Snider.
Acknowledgments
Researchers received funding from the National Institutes of Health, the University of North Carolina Department of Cell Biology and Physiology, the National Science Foundation, the United Leukodystrophy Foundation, and Elise's Corner Fund.
Return to top of page.
| |
|
Nov 25 2019 Fetal Timeline Maternal Timeline News
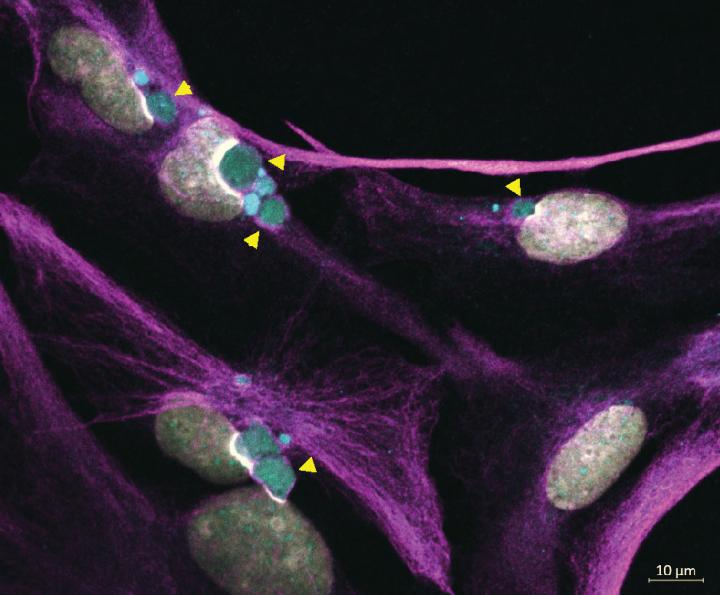 Immunofluorescence staining of Alexander Disease iPSC-astrocytes showing: WHITE = Cell Nuclei; MAGENTA = cytoplasmic GFAP filaments; and GREEN = perinuclear GFAP aggregates indicated by yellow arrowheads. CREDIT Lab of Natasha Snider PhD, UNC School of Medicine.
|



