|
|
Developmental Biology - Neuron Development
Mutated Neuron Antennae Impair Brain Connections
Defective cilia can affect connectivity between neurons in brain development causing rare neurodevelopmental conditions like Joubert syndrome and related disorders...
UNC School of Medicine scientists led by Eva Anton PhD, find mutations in primary cilia can cause rare neurodevelopmental disorders like Joubert syndrome and related disorders.
Axons are the long thread-like extensions of neurons that send electrical signals to other brain cells. Thanks to axonal connectivity, our brains and bodies can do all necessary tasks. Even before we're born, we need axons to grow in tracts throughout gray matter and connect properly as our brains develop. UNC School of Medicine researchers have now found a key reason why connectivity goes awry and leads to rare but debilitating neurodevelopmental conditions.
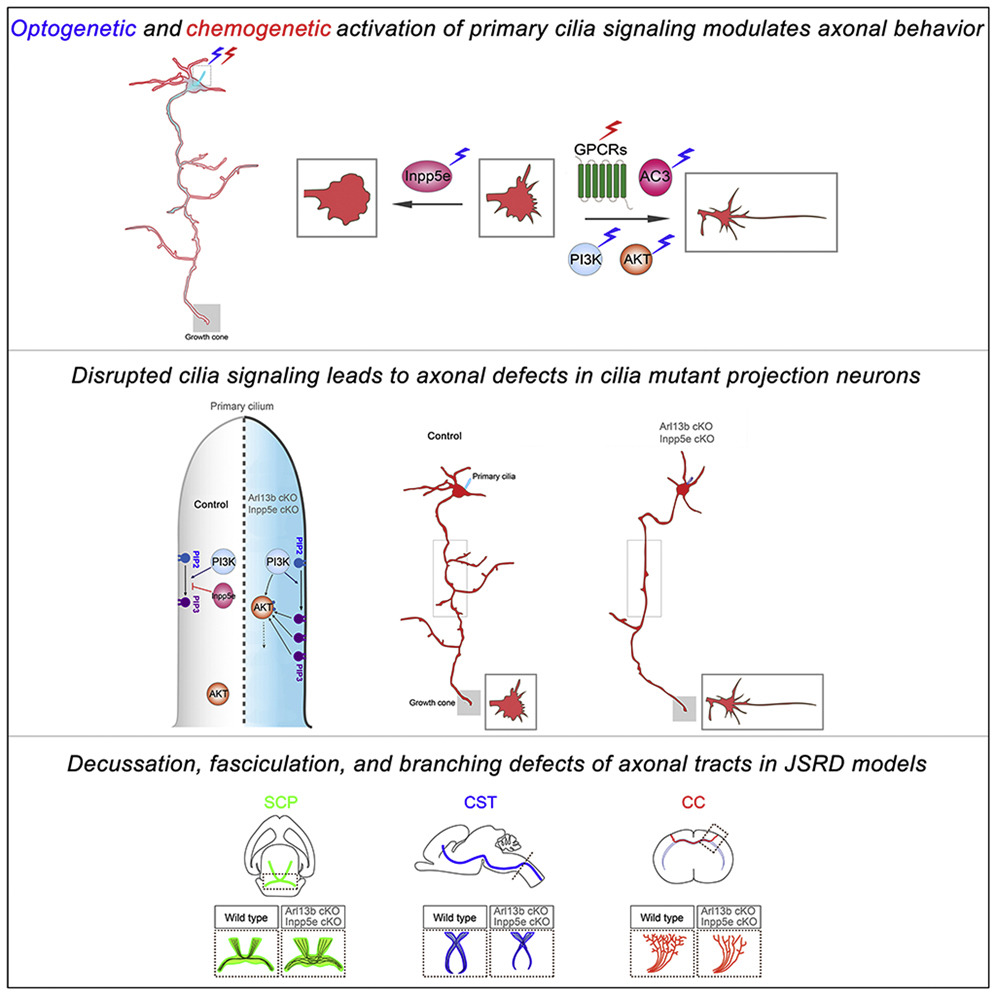
Published in the journal Developmental Cell, researchers led by Eva Anton PhD, professor of cell biology and physiology at UNC-Chapel Hill, show how two gene mutations alter the function of neuronal cilia - antennae-like protuberances found on many cell types. The resulting dysfunctional cilia affect axonal connectivity and leads to rare Joubert syndrome-related disorders (JSRD).
"Our experiments demonstrate that ciliary signaling facilitates appropriate patterns of axon tract development and connectivity. Disrupting ciliary signaling can lead to axonal tract malformations in JSRD."
Eva S. Anton PhD, University of North Carolina, Neuroscience Center and the Department of Cell and Molecular Physiology, University of North Carolina School of Medicine, Chapel Hill, North Carolina, USA.
Although cilia are found on most cell types, their significance in brain development, has been largely underappreciated, until recently. Scientists now know that cilia sense the environment around them, and dysfunctional cilia mess up axonal growth and connectivity during fetal development.
Babies born with dysfunctional cilia and associated irregular axonal growth and connectivity can develop JSRD. Molar tooth sign, a characteristic defect of axonal projections detectable in brain MRI images, is often used to diagnose JSRD.
People with the condition experience developmental delays, intellectual disabilities, abnormal respiratory rhythms, trouble controlling their body movements, and other serious health issues. But how this happens has not been clear.
Using neuron-specific mouse genetic models of two genes called Arl13b and Inpp5 and related human mutations from JSRD patients, as well as chemo-genetic and opto-genetic manipulation of primary cilia signaling, Anton and colleagues investigated how cilia become dysfunctional and affect axonal connectivity during brain development.
In mice, they found that deletion of Arl13b or Inpp5e impairs the ability of the primary cilium to function as a signaling hub, thus allowing them to examine how cilia-driven signaling regulates axon growth and connectivity in normal and JSRD brains. Anton and colleagues went on to delineate ciliary-driven changes in cell signaling, particularly the ones mediated through major signaling proteins PI3K AKT, and AC3 effectively modulate axonal behavior.
Before this research, the significance of primary cilia in the emergence of brain connectivity were undefined. Nor did the research community understand exactly how cilia dysregulation led to axonal tract defects in Joubert syndrome-related disorders.
"By shedding light on the significance of primary cilia in the emergence of brain connectivity, this research helps us understand how cilia dysregulation led to axonal tract defects in Joubert syndrome-related disorders. Our studies indicate precise manipulation of ciliary signaling in the future may be tested and utilized to alleviate neuronal connectivity defects in ciliopathies, such as JSRD."
Eva S. Anton PhD.
Abstract
Highlights
• Chemogenetic and/or optogenetic activation of primary cilia alters axonal behavior
• Ciliary activity modulates axonal growth cones and filopodial-lamellipodial balance
• Arl13b-Inpp5e activity in cilia facilitates axonal tract formation and targeting
• Disrupted ciliary signaling contributes to axonal tract malformations in JSRD
Summary
Appropriate axonal growth and connectivity are essential for functional wiring of the brain. Joubert syndrome-related disorders (JSRD), a group of ciliopathies in which mutations disrupt primary cilia function, are characterized by axonal tract malformations. However, little is known about how cilia-driven signaling regulates axonal growth and connectivity. We demonstrate that the deletion of related JSRD genes, Arl13b and Inpp5e, in projection neurons leads to de-fasciculated and misoriented axonal tracts. Arl13b deletion disrupts the function of its downstream effector, Inpp5e, and deregulates ciliary-PI3K/AKT signaling. Chemogenetic activation of ciliary GPCR signaling and cilia-specific optogenetic modulation of downstream second messenger cascades (PI3K, AKT, and AC3) commonly regulated by ciliary signaling receptors induce rapid changes in axonal dynamics. Further, Arl13b deletion leads to changes in transcriptional landscape associated with dysregulated PI3K/AKT signaling. These data suggest that ciliary signaling acts to modulate axonal connectivity and that impaired primary cilia signaling underlies axonal tract defects in JSRD.
.
Authors
Jiami Guo, James M. Otis, Sarah K. Suciu, Christy Catalano, Lei Xing, Sandii Constable, Dagmar Wachten, Stephanie Gupton, Janice Lee, Amelia Lee, Katherine H. Blackley, Travis Ptacek, Jeremy M. Simon, Stephane Schurmans, Garret D. Stuber, Tamara Caspary and E.S. Anton.
Acknowledgments
This research was funded by the National Institutes of Health and the Natural Sciences and Engineering Research Council of Canada.
Return to top of page.
| |
|
Dec 20 2019 Fetal Timeline Maternal Timeline News
 Axonal growth and connectivity are essential to functional wiring in the brain. Above, disrupted crisscrossing and organization of axons (yellow) reflect deletion of gene, Arl13b specific to Joubert syndrome.
CREDIT Anton Lab.
|




