|
|
Developmental Biology - Gene Manipulation
Embedding A Special Molecular Switch Into Genes
A special molecular switch may be able to be embedded into genes allowing doctors to control gene dosing...
Scientists at Scripps Research in Jupiter, Florida have developed a special molecular switch that could be embedded into genes to allow doctors to control gene therapies. The feat is reported in the scientific journal Nature Biotechnology.
Gene therapy designers offer what may be the first viable technique for adjusting the level of activity in therapeutic genes. The lack of such a basic safety feature has limited development of gene therapy, which otherwise holds promise for intervention in genetically based 'diseases'. The technique appears to solve a major safety issue and may lead to more use of gene treatment strategies.
The Scripps Research team, led by principal investigator Michael Farzan PhD, demonstrated the power of their new switching technique by incorporating it into a gene that produces the hormone erythropoietin — used in the treatment of anemia.
The scientists showed they could reduce the function of its gene to very low levels by targeting a special molecule embedded within it. They can also increase the gene's function, over a wide range, using injected control molecules called morpholinos. The U.S. Food and Drug Administration has already declared morpholinos safe to use in other applications.
"I think that our approach offers the only practical way at present to regulate gene therapy in an animal or a human."
Michael Farzan PhD, Department of Immunology and Microbiology, The Scripps Research Institute, Jupiter, Florida, USA.
Gene therapies work by inserting copies of a therapeutic gene into the cells of a patient born without functional copies of that gene. The strategy has long been seen as having enormous potential to cure disease caused by defective genes.
It also could enable the steady, long-term delivery to patients of therapeutic molecules impractical to deliver in pills or by injections. However, such therapies have been viewed as inherently risky as once they are delivered to a patient's cells — they can't be switched off or modified. As a result, only a handful of gene therapies are FDA-approved to date.
According to Farzan, the simplicity of the technique, and the fact that morpholinos are already FDA-approved, could allow the new transgene switching system to be used in a wide variety of gene therapies.
Farzan's team, including study co-first authors Guocai Zhong PhD and Haimin Wang a postdoctoral researcher in the Farzan lab, crafted a transgene switch from a family of ribonucleic acid (RNA) molecules called hammerhead ribozymes. These remarkable ribozymes have the ability to cut themselves in two as soon as copied into RNA by the DNA that encodes them. A therapeutic transgene containing DNA of this ribozyme can be copied into strands of RNA, called transcripts, that tend to separate into two pieces before being translated into proteins.
However, this self-cleaving action of the ribozyme can be blocked by RNA-like morpholinos that latch onto the ribozyme's active site. If this happens, the transgene transcript will remain intact and will be more likely to be translated into the therapeutic protein.
The ribozyme effectively acts as an "off switch" for the transgene. Whereas the matching morpholinos injected into the tissue with the transgene, "turn on" the transgene back on - to a degree dependent on the morpholino dose.
Scientists tested a hammerhead ribozyme called N107 used as an RNA switch in prior studies. Over months of experimentation they were able to modify the ribozyme until it had a dynamic range that was dozens of times more reliable.
They then demonstrated the switch in a mouse model of an EPO gene therapy, not yet FDA-approved but considered potentially better than current methods for treating anemia associated with severe kidney disease. Injecting an EPO transgene into muscle tissue in live mice, they showed the embedded ribozyme suppressed EPO production to a very low level.
Injecting a small dose of the morpholino molecules into the same affected tissue strongly reversed that suppression, allowing EPO production to rise by a factor of more than 200 — a result that remained stabile for more than a week, compared to a few hours for EPO delivered by a standard injection. Those properties make the ribozyme-based switch potentially suitable for real clinical applications.
Farzan and his colleagues are now working to adapt their ribozyme switch technique to be used as a gene therapy failsafe mechanism, deactivating errant transgenes permanently.
Abstract
Widespread use of gene therapy technologies is limited in part by the lack of small genetic switches with wide dynamic ranges that control transgene expression without the requirement of additional protein components1,2,3,4,5. In this study, we engineered a class of type III hammerhead ribozymes to develop RNA switches that are highly efficient at cis-cleaving mammalian mRNAs and showed that they can be tightly regulated by a steric-blocking antisense oligonucleotide. Our variant ribozymes enabled in vivo regulation of adeno-associated virus (AAV)-delivered transgenes, allowing dose-dependent and up to 223-fold regulation of protein expression over at least 43 weeks. To test the potential of these reversible on-switches in gene therapy for anemia of chronic kidney disease6, we demonstrated regulated expression of physiological levels of erythropoietin with a well-tolerated dose of the inducer oligonucleotide. These small, modular and efficient RNA switches may improve the safety and efficacy of gene therapies and broaden their use.
Authors
Guocai Zhong, Haimin Wang, Wenhui He, Yujun Li, Huihui Mou, Zachary J. Tickner, Mai H. Tran, Tianling Ou, Yiming Yin, Huitian Diao and Michael Farzan.
Acknowledgments
This work was supported by NIH R37 AI091476, NIH AI1129868 and NIH DP1 DA043912 (M.F.).
Return to top of page.
| |
|
Dec 30 2019 Fetal Timeline Maternal Timeline News
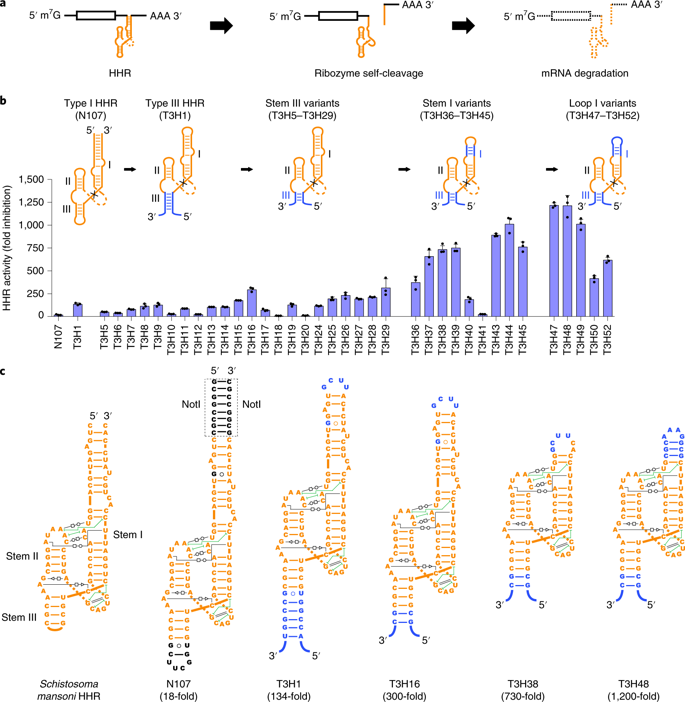 Gene therapies have been seen as risky because they cannot be switched off or modified after placement into a patient's genes. Only a handful of gene therapies are FDA-approved. Morpholinos are already FDA-approved, and in the near future, could allow transgene switching in a wide variety of gene therapies. CREDIT Scripps Research.
|



