|
|
Developmental Biology - Male Infertility
Condensing DNA to Fit Into Sperm Can Lead to Errors
Researchers track defective DNA errors in sperm and potentially find ways to correct defects...
For the past decade, research has linked infertility to defective sperm which fail to "evict histone proteins" from strands of DNA [nucleosomes
] during fetal development. However, what mechanisms may have failed to evict histones and where these "failures" happens in sperm DNA, is controversial and unclear.
Researchers at Penn Medicine, using new genome-wide DNA sequencing tools, reveal precise gene locations where histones exist and a key gene that regulates DNA sequencing in sperm.
The findings are published in Developmental Cell.
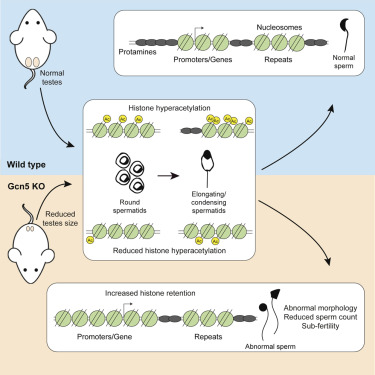
Going a step further, researchers created a mouse model carrying a mutated version of the gene Gcn5 — allowing investigators to closely track sperm defects from early sperm development through fertilization and onward. This step could potentially lead to understanding not only infertility and ways to reverse it, but how epigenetic mutations get passed to the embryo from males either naturally or through in vitro fertilization.
Epigenetics are factors influencing an organism's genetics that are not encoded in DNA, but play a strong role in sperm and egg formation.
"For men who have unexplained infertility, everything may look normal at the doctors: normal semen counts, normal motility, yet he still has problems conceiving. One explanation might be histones are in the wrong location, which may affect sperm and then early development.
Now, we have a really good model to study what happens when you don't rid histones appropriately from sperm and what that may look like in the embryo."
Lacey J. Luense PhD, Research Associate, Berger laboratory.
Healthy sperm lose 90 to 95 percent of histones, the main proteins in chromatin that package DNA and turn genes on and off. Histones are replaced with protamines, which are smaller proteins than histones and can better pack DNA into tiny sperm. Given the role of retained histones in infertility and later in embryonic development, there is great interest in determining histone to gene location so these locations can be identified for their potential affect and, if needed, possible treatment.
Past studies have produced conflicting results on the whereabouts of histones.
A technology known as MNase-sequencing uses an enzymatic reaction to pinpoint histone location. This placement reveals if retained histones exist at important gene promotor regions. Other studies using this enzymatic approach have found histones at DNA repeats or placed in so-called "gene deserts" where histones have less of a role in gene regulation.
"There is controversy in the field trying to understand these discrepant points of data. In the new study, we found that both of these previously described models are correct.
We find histones on genes that appear to be important for embryo development, but we also find them at repetitive [gene] elements, where they need to be turned off to prevent expression/function of these regions in the embryo."
Lacey J. Luense PhD.
Researchers applied a technology known as ATAC-sequencing, a precise and fast approach, to track waves of histones at unique sites across the genome during the early and late stages of mouse sperm development. ATAC-seq identified where the mouse genome was open or closed [functioning or not functioning] - particularly those regions that retained sperm histones — make a cut and tag the DNA to be sequenced revealing its function.
In mice created with the mutated Gcn5 gene, researchers found the mice to have very low fertility. They also found that retained histones in normal mouse sperm correlated with histone positions in very early embryos. This observation supports the hypothesis that paternal histones transfer epigenetic information to the next generation..
"Right now, the burden of IVF and other assisted-reproductive technologies fall on women. Even if the male factor is infertile, women still have to go through hormone injections and additional procedures.
Imagine being able to apply epigenetic therapeutics to change the levels of histones and protamines in males before embryogenesis? That's one of the questions we want to explore and this model will allow us to move toward that direction."
Shelley L. Berger PhD, the Daniel S. Och PhD and Professor, Departments of Cell and Developmental Biology, Director of the Penn Epigenetics Institute and senior author.
There are numerous available epigenetic drugs used to treat cancer and other diseases. Given their mechanisms, treating sperm with drugs to increase histone eviction is one potential route to explore.
Limitations with human embryos in science have led to a lack of overall research on infertility and the role of the father's epigenome on embryo development, which underscores the importance of studies such as this, say the researchers.
"A lot ofdifferent factors can alter the sperm epigenome, for example diet, drugs and alcohol. We are just now begining to understand how these factors can affect the child and its development.
These initial, basic studies are critical to better understand what drives epigenetic mutations."
Lacey J. Luense PhD.
Highlights
• ATAC-seq localizes retained nucleosomes to promoters and repetitive DNA found in sperm
• Gcn5-mediated histone acetylation is necessary for proper spermiogenesis
• Gcn5 loss alters chromatin dynamics leading to increased histone retention in sperm
• Gcn5 is necessary for normal sperm formation and male fertility in mice
Summary
During mammalian spermatogenesis, germ cell chromatin undergoes dramatic histone acetylation-mediated reorganization, whereby 90%–99% of histones are evicted. Given the potential role of retained histones in fertility and embryonic development, the genomic location of retained nucleosomes is of great interest. However, the ultimate position and mechanisms underlying nucleosome eviction or retention are poorly understood, including several studies utilizing micrococcal-nuclease sequencing (MNase-seq) methodologies reporting remarkably dissimilar locations. We utilized assay for transposase accessible chromatin sequencing (ATAC-seq) in mouse sperm and found nucleosome enrichment at promoters but also retention at inter- and intragenic regions and repetitive elements. We further generated germ-cell-specific, conditional knockout mice for the key histone acetyltransferase Gcn5, which resulted in abnormal chromatin dynamics leading to increased sperm histone retention and severe reproductive phenotypes. Our findings demonstrate that Gcn5-mediated histone acetylation promotes chromatin accessibility and nucleosome eviction in spermiogenesis and that loss of histone acetylation leads to defects that disrupt male fertility and potentially early embryogenesis.
Authors
Lacey J. Luense, Greg Donahue, Enrique Lin-Shiao, Richard Range, Angela H. Weller, Marisa S. Bartolomei and Shelley L. Berger.
Acknowledgments
The University of Pennsylvania Health System's patient care facilities include: the Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center, recognized as one of the nation's top "Honor Roll" hospitals by U.S. News & World Report.
Return to top of page.
| |
|
Jan 14 2020 Fetal Timeline Maternal Timeline News
 A new mouse model allows researchers to track defective sperm and potentially find ways to correct infertility. CREDIT Penn Medicine
|



