|
|
Developmental Biology - Immune System
One Infection Can Induce Irritable Bowel Syndrome
Intestinal infection is just the beginning for IBS...
Sometimes the end of an intestinal infection is just the beginning of a lot more misery. Of those who contract traveler's diarrhea, for example, an unlucky few go on to develop irritable bowel syndrome (IBS), a chronic inflammation of the intestinal tract. This new research appears in the journal CELL.
Scientists aren't sure exactly how, but think an infection may contribute to IBS by damaging the gut nervous system. A new Rockefeller University study takes a close look at why neurons in the gut die and how the immune system normally protects them.
Conducted with mice, the experiments offer insight on IBS and point toward potential new treatments.
Keeping inflammation in check
In a healthy gut, the immune system strikes a careful balance between responding to threats and controlling that response to avoid damage.
"Inflammation helps the gut ward off an infection. But, too much of it can cause lasting harm. Our work explores the complex mechanisms that prevent inflammatory responses from destroying neurons."
Daniel Mucida PhD, Associate Professor and head of the Laboratory of Mucosal Immunology, Rockefeller University, New York, New Yoek, USA.
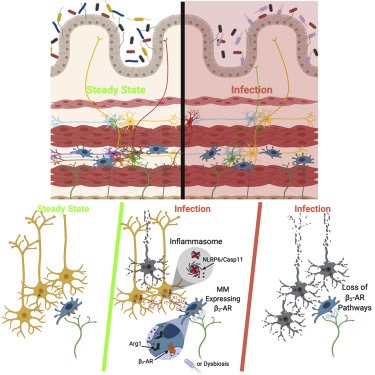
To understand the effects of an infection on the nervous system, Mucida and colleagues gave mice a weakened form of Salmonella, a bacterium that causes food poisoning. Afterwards, analyzing the effects on neurons within the intestine. They found salmonella infection induced a long-lasting reduction in neurons. They attribute this effect to the expression/function of two genes, Nlrp6 and Caspase 11, which contribute to a specific inflammatory response.
This response can ultimately prompt cells to undergo a type of programmed cell death. When researchers manipulated mice and eliminated these genes - specifically in neurons - the number of dead neurons decreased.
"This mechanism of cell death has been documented in other types of cells, but never before in neurons. We believe these gut neurons may be the only ones to die this way."
Fanny Matheis, graduate student, Laboratory of Mucosal Immunology, Rockefeller University, New York, New Yoek, USA.
Macrophages to The Rescue
Although it's not clear exactly how inflammation causes neurons to commit cell suicide, scientists already have clues suggesting it might be possible to interfere with the process. The key may be a specialized set of gut immune cells, known as muscularis macrophages.
Previous work in Mucida's lab has shown that muscularis macrophage cells express inflammation-fighting genes and collaborate with neurons to keep food moving through the digestive tract. If these neurons die off due to infection, a possible result is constipation — one of a number of IBS symptoms.
The team demonstrates in their report how macrophages ameliorate constipation. Macrophages possess a specific receptor molecule that receives stress signals released by neurons in response to infection. Activated, this receptor prompts macrophages to produce molecules called polyamines, which the scientists think interfere with the cell death process.
Getting Back to Normal
In other experiments researchers found Salmonella infection alters the community of microbes within the guts of mice. But when restored back to normal, the animals' neurons recovered.
"Using what we've learned about macrophages, one could think about ways to disrupt the inflammatory process killing neurons," says Paul Muller, a postdoctoral fellow in the lab.
It might be possible to develop better treatments for IBS by boosting polyamine production — through diet or by restoring gut microbes. As short-term stress responses also appear to have a protective effect, Muller thinks it may be helpful to also target that system.
Highlights
• Enteric pathogens trigger reversible neuronal loss and long-term GI symptoms
• Enteric infection-triggered neuronal loss is Nlrp6- and caspase 11-dependent
• Intestinal muscularis macrophages (MMs) rapidly respond to enteric pathogens
• Neuronal death is limited by a MM-? 2-adrenergic-arginase 1-polyamine axis
Summary
Enteric-associated neurons (EANs) are closely associated with immune cells and continuously monitor and modulate homeostatic intestinal functions, including motility and nutrient sensing. Bidirectional interactions between neuronal and immune cells are altered during disease processes such as neurodegeneration or irritable bowel syndrome. We investigated the effects of infection-induced inflammation on intrinsic EANs (iEANs) and the role of intestinal muscularis macrophages (MMs) in this context. Using murine models of enteric infections, we observed long-term gastrointestinal symptoms, including reduced motility and loss of excitatory iEANs, which was mediated by a Nlrp6- and Casp11-dependent mechanism, depended on infection history, and could be reversed by manipulation of the microbiota. MMs responded to luminal infection by upregulating a neuroprotective program via ? 2-adrenergic receptor (? 2-AR) signaling and mediated neuronal protection through an arginase 1-polyamine axis. Our results identify a mechanism of neuronal death post-infection and point to a role for tissue-resident MMs in limiting neuronal damage..
Authors
Fanny Matheis, Paul A. Muller, Christina L. Graves, Ilana Gabanyi, Zachary J. Kerner, Diego Costa-Borges, Tomasz Ahrends, Philip Rosenstiel and Daniel Mucida.
Return to top of page.
| |
|
Jan 16 2020 Fetal Timeline Maternal Timeline News
 close to inflammatory molecule Nlrp6 (pink).jpg) Along the edge of the small intestine, neurons (GREEN) appear in close proximity to the inflammatory molecule Nlrp6 (PINK).
CREDIT
Laboratory of Mucosal Immunology
|

 close to inflammatory molecule Nlrp6 (pink).jpg)


