|
|
Developmental Biology - Gastrointestinal System
Intestinal Organoids Developed to Model Disease
A new way to generate human cells for testing gastrointestinal diseases...
Published in Nature Communications, a novel process improves drug screening, uncovering new therapies for treating a variety of intestinal diseases, such as inflammatory bowel disease, colon cancer and Cystic Fibrosis.
Researchers at the Center for Regenerative Medicine (CReM) of Boston University and Boston Medical Center use donated Human Induced Pluripotent Stem Cells (hiPSCs), and reprogrammed them into a primitive state.
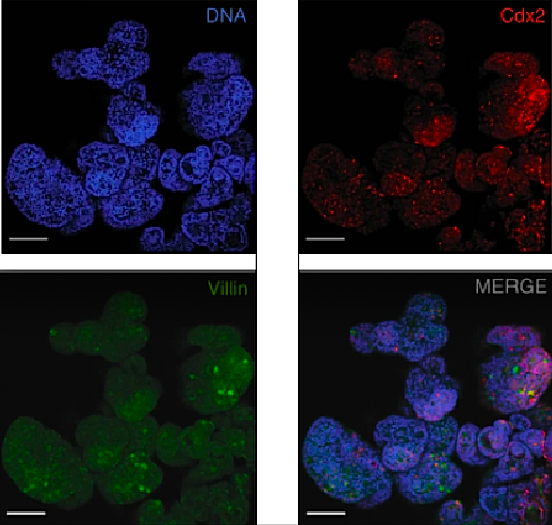
These hiPSCs cells were then pushed to differentiate into intestinal cells using growth factors which stimulated them to become organoids growing in a petri dish.
This new protocol allows the cells to develop without a mesenchyme or middle layer, which typically supports the growth of intestinal epithelial cells.
By taking out the mesenchyme, researchers could exclusively study epithelial cells that make up the intestinal tract.
Using CRISPR technology, researchers were then able to modify and create a novel iPSC stem cell line that glowed green when the cells differentiated into intestinal cells. This allowed researchers to follow each process in cell differentiation.
"Generating organoids in our lab allows us to create more accurate disease models, which are then used to test treatments and therapies targeted to a specific genetic defect or tissue. All of this possible without harming the patient. This approach allows us to determine what treatments could be more effective, and which are ineffective against a disease."
Gustavo Mostoslavsky MD, PhD, Co-Director CReM; Associate Professor of Medicine and Microbiology, Boston University School of Medicine - Gastroenterology, Boston Medical Center, Boston Massachusetts, USA.
Using this new protocol, researchers generated intestinal organoids from iPSCs containing a mutation causing Cystic Fibrosis (CF). CF typically affects several organs, gastrointestinal tract included.
CRISPR technology was then used to correct the CF mutation in the intestinal organoids. Intestinal organoids with the mutation did not respond to a known CF drug. But, genetically corrected organoids did. So, the new procedure demonstrates organoids have potential as disease models and in therapeutic screenings, to test corrective procedures.
The new protocol developed in this study provides strong evidence for continued use of human iPSCs to study cell development and tissue engineering. Possibly advancing the field of regenerative medicine.
"I hope this study helps move forward our collective understanding about how diseases impact the gastrointestinal tract at the cell level.
Continued development of novel techniques to create highly differentiated cells, useful for developing disease models in a lab, will pave the way for development of more targeted approaches to treat many different diseases."
Gustavo Mostoslavsky MD, PhD.
Abstract
Efficient generation of human induced pluripotent stem cell (hiPSC)-derived human intestinal organoids (HIOs) would facilitate the development of in vitro models for a variety of diseases that affect the gastrointestinal tract, such as inflammatory bowel disease or Cystic Fibrosis. Here, we report a directed differentiation protocol for the generation of mesenchyme-free HIOs that can be primed towards more colonic or proximal intestinal lineages in serum-free defined conditions. Using a CDX2eGFP iPSC knock-in reporter line to track the emergence of hindgut progenitors, we follow the kinetics of CDX2 expression throughout directed differentiation, enabling the purification of intestinal progenitors and robust generation of mesenchyme-free organoids expressing characteristic markers of small intestinal or colonic epithelium. We employ HIOs generated in this way to measure CFTR function using cystic fibrosis patient-derived iPSC lines before and after correction of the CFTR mutation, demonstrating their future potential for disease modeling and therapeutic screening applications.
Authors
Aditya Mithal, Amalia Capilla, Dar Heinze, Andrew Berical, Carlos Villacorta-Martin, Marall Vedaie, Anjali Jacob, Kristine Abo, Aleksander Szymaniak, Megan Peasley, Alexander Stuffer, John Mahoney, Darrell N. Kotton, Finn Hawkins and Gustavo Mostoslavsky.
Acknowledgments
The authors would like to thank Seonmi Park and Greg Miller for their invaluable technical support. We thank Brian Tilton and the BUMC Flow Cytometry Core for their assistance in cell sorting and flow cytometry experiments, supported by NIH grant 1UL1TR001430, as well as Marianne James and the Boston University iPSC Core for the karyotyping and banking of BU1CG. A.M. is supported by 5T32HL007969-12; A.C., A.J., K.A., and D.H. by TL1TR001410; A.B. by T32HL007035; D.N.K. by R01HL095993 and R01HL128172; F.H. by Cystic Fibrosis Foundation grant HAWKIN15XX0 and NIH grant R01HL139799; and G.M. by R01AI130199 and 1R21NS111499-01. The CReM iPSC Core is supported by NIH 1R24HL123828-01 and U01TR001810.
About Boston Medical Center
Boston Medical Center is a private, not-for-profit, 514-bed, academic medical center that is the primary teaching affiliate of Boston University School of Medicine. It is the largest and busiest provider of trauma and emergency services in New England. Boston Medical Center offers specialized care for complex health problems and is a leading research institution, receiving more than $97 million in sponsored research funding in fiscal year 2018. It is the 15th largest funding recipient in the U.S. from the National Institutes of Health among independent hospitals. In 1997, BMC founded Boston Medical Center Health Plan, Inc., now one of the top ranked Medicaid MCOs in the country, as a non-profit managed care organization. Boston Medical Center and Boston University School of Medicine are partners in Boston HealthNet - 14 community health centers focused on providing exceptional health care to residents of Boston. For more information, please visit http://www.bmc.org.
Return to top of page.
| |
|
Jan 21 2020 Fetal Timeline Maternal Timeline News
 Organoids at day 85. Each made from BU3NGST NKX2-1GFPneg cell outgrowth; each producing fluorescent labels at separate emission wavelengths. (scale bar = 50 ym) CREDIT Laboratory of Mucosal Immunology.
|



