|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
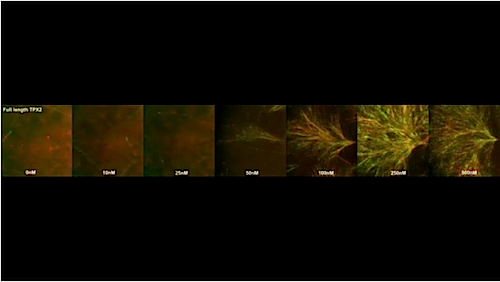
Developmental Biology - Cell Division Princeton Recreates A Key Step In Cell Division • One key finding from the study is that, like augmin, TPX2 can bind to microtubules and recruit y-TuRC to initiate branching microtubule nucleation. In the Nature Communications paper, Petry and her former graduate student Matthew King further reveal that TPX2 behaves like a liquid in promoting branching microtubule nucleation. Specifically, TPX2 forms a liquid layer on the surface of existing microtubules that beads up into tubulin-containing droplets, much like morning dew on spider webs. Researchers found that TPX2 and tubulin can condense together to form liquid-like droplets through a phase-separation mechanism identical to the one that causes oil droplets to form in water. New microtubules can form from these TPX2-tubulin droplets and, because the droplets condense on the surface of existing microtubules, this results in the formation of branched microtubule structures. "The study suggests that the co-condensation of TPX2 and tubulin creates a local reservoir of tubulin on a pre-existing microtubule that may be necessary to efficiently promote branching microtubule nucleation." Together, both studies reveal that TPX2, somewhat overlooked before, is the lynchpin of branching microtubule nucleation. It travels to microtubules first to assemble all of the other components that ultimately give rise to a branching microtubule, and it does this while behaving like a liquid. Petry and colleagues think that cellular signals may regulate TPX2 condensation to ensure that it only occurs when a cell is dividing and needs to form a spindle. "Our findings on the behavior of TPX2 may guide future therapeutic efforts aimed at modulating cell division. In addition, our reconstitution of branching microtubule nucleation is an important step toward reconstituting the entire spindle apparatus, as well as other cellular structures that depend on this microtubule assembly pathway." Abstract Nature Communications Phase separation of substrates and effectors is proposed to enhance biological reaction rates and efficiency. Targeting protein for Xklp2 (TPX2) is an effector of branching microtubule nucleation in spindles and functions with the substrate tubulin by an unknown mechanism. Here we show that TPX2 phase separates into a co-condensate with tubulin, which mediates microtubule nucleation in vitro and in isolated cytosol. TPX2-tubulin co-condensation preferentially occurs on pre-existing microtubules, the site of branching microtubule nucleation, at the endogenous and physiologically relevant concentration of TPX2. Truncation and chimera versions of TPX2 suggest that TPX2-tubulin co-condensation enhances the efficiency of TPX2-mediated branching microtubule nucleation. Finally, the known inhibitor of TPX2, the importin-a/ß heterodimer, regulates TPX2 condensation in vitro and, consequently, branching microtubule nucleation activity in isolated cytosol. Our study demonstrates how regulated phase separation can simultaneously enhance reaction efficiency and spatially coordinate microtubule nucleation, which may facilitate rapid and accurate spindle formation. Authors Matthew R. King and Sabine Petry. Acknowledgments The work published in both papers was supported by the National Institutes of Health (NIH), the Pew Scholars Program in the Biomedical Sciences, and the David and Lucile Packard Foundation. The work published in eLife was additionally supported by a Howard Hughes Medical Institute Gilliam Fellowship, a National Science Foundation Graduate Research Fellowship, and an American Heart Association predoctoral fellowship. The authors thank members of the Petry Lab for helping with this work, including Ray Alfaro-Aco, Michael Rale, Mohammad Safari, Akanksha Thawani, and Sagar Setru. We are especially grateful to Cliff Brangwynne for experimental suggestions and the Petry Lab, Ibrahim Cisse, and Kassandra Ori-McKenney for feedback on the manuscript. This work was supported by a Ph.D. training grant T32GM007388 by NIGMS of the National Institutes of Health (to M.R.K.), as well as the New Innovator Award of NIGMS of the National Institutes of Health (DP2), the Pew Scholars Program in the Biomedical Sciences, and the David and Lucile Packard Foundation (all to S.P.). Return to top of page. | Jan 22 2020 Fetal Timeline Maternal Timeline News 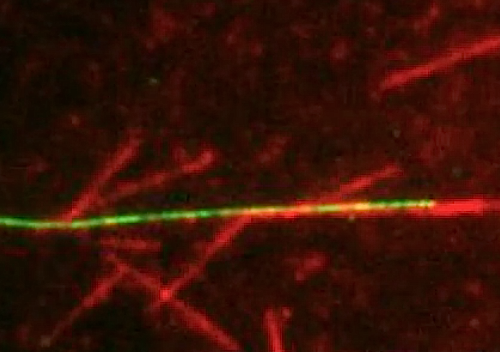 In the experiment, new microtubules formed when TPX2 condensed with tubulin into liquid-like droplets. CREDIT Raymundo Alfaro-Aco, Sabine Petry, Princeton University
|
||||||||||||||||||||||||||||