|
|
Developmental Biology - Autism Spectrum
First Induced Autism Symptom Improvements
Bumetanide - a drug for edema - improves symptoms in young children with autism without significant side effects...
Bumetanide - a prescription drug for edema (the build-up of fluid in the body) - improves some of the symptoms in young children with autism spectrum disorders and has no significant side effects, according to a new study from multiple research institutions in China along with the University of Cambridge, Cambridge, UK published in Translational Psychiatry.
The study demonstrates for the first time that a drug can improve Autism symptoms by decreasing the ratio of GABA to glutamate. Both are neurotransmitters or chemical messengers that help brain nerve cells communicate.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder estimated to affect one in every 160 children worldwide. It is characterised by impairments in social communication, manifesting as problems in understanding emotions, and in using non-verbal communication such as eye contact and smiling. Failure to develop these subtle skills can lead to an inability to maintain and understand social relationships. People with ASD tend to have restricted interests and repetitive behaviors, but in mild cases, can live independently. However, some conditions can be severe and require life-long assistance.
Although the biological mechanics of ASD are still largely unknown, previous research has suggested it may result from changes in brain development early in life. In particular regarding GABA, a brain neurotransmitting chemical controlling nerve cell communication.
In an adult, GABA inhibits neurotransmissions — it switches nerve cells 'off'. In fetal life and early postnatal development, GABA is mostly excitatory and switches nerve cells 'on' making them fire.
Alterations in the timing of the release of GABA (from excitatory to inhibitory) can cause a delay in when and how developing neural circuits mature and function, thus affecting neural network activity. This Implies that intervention at an early age may help reduce some symptoms that can make life challenging for people with ASD.
Current ASD treatment for preschoolers is mainly behavioral intervention, such as using play and guided activities between parents and their child to boost language, social and cognitive skills. However, with limited resources there is an inequality in access to these treatments across the globe, particularly in developing countries.
Previous studies in rats and small clinical trials involving children with ASD suggested the drug bumetanide — approved for use in edema, a condition that results in a build-up of fluid in the body — might help reduce symptoms of ASD. ASD can be reliably diagnosed by age 24 months or even as early as 18 months.
Now, an international collaboration between institutions across China and the University of Cambridge in the UK, shows that bumetanide is safe to use and effective at reducing symptoms in children as young as three years old.
The team recruited 83 children aged three to six years old, dividing them into two groups. One group of 42 children received 0.5mg of bumetanide twice a day for three months. A control group of 41 children received no treatment. Researchers assessed symptoms using the Childhood Autism Rating Scale (CARS), used to rate behavior such as imitation, emotional response and verbal and non-verbal communication. Children scoring above 30 on the scale are considered to have ASD.
Before treatment, both groups had similar CARS scores, but afterwards, the treatment group now had a mean total score of 34.51 (compared to the control group mean score of 37.27). Importantly, the treatment group showed a significant reduction in the number of items on the CARS assigned a score greater than or equal to three, with the average number of 3.52 items in the treatment group compared to 5.49 items in the control group.
"I have many children with ASD under my care, but as psychological treatment resources are not available in many places, we are unable to offer them treatment. An effective and safe treatment will be very good news for them.
"The mother of a four year old boy living in a rural area outside Shanghai who received the treatment told me that he was now better at making eye contact with family members and relatives and was able to participate more in activities. In the future, we hope to be able to ensure all families, regardless of where they are living, can receive treatment for their child."
Fei Li PhD, Shanghai Institute of Pediatric Research, Xinhua Hospital, Shanghai, Jiao Tong University School of Medicine, Shanghai, China, and clinical leader of the study.
To understand the mechanisms underlying these improvements, researchers used magnetic resonance spectroscopy to image brain concentrations of neurotransmitters. They found in two key brain regions - the insular cortex (which plays a role in emotions, empathy and self-awareness) and visual cortex (responsible for integrating and processing visual information) - the ratio of GABA to glutamate decreased over the three-month period in the treatment group. GABA and glutamate are known to be important for brain plasticity and promoting learning.
The team hopes the discovery that bumetanide changes the concentration of GABA to glutamate will provide a useful biomarker of how effective a treatment will be. However, they caution further research is needed to confirm the effectiveness of bumetanide as a treatment for ASD.
Abstract
Bumetanide has been reported to alter synaptic excitation–inhibition (E-I) balance by potentiating the action of ?-aminobutyric acid (GABA), thereby attenuating the severity of autism spectrum disorder (ASD) in animal models. However, clinical evidence of its efficacy in young patients with ASD is limited. This was investigated in the present clinical trial of 83 patients, randomised to the bumetanide group (bumetanide treatment, 0.5 mg twice daily) or the control group (no bumetanide treatment). Primary [Children Autism Rating Scale (CARS)], secondary [Clinical Global Impressions (CGI)], and exploratory [inhibitory (?-aminobutyric acid, GABA) and excitatory (glutamate, Glx) neurotransmitter concentrations measured in the insular cortex (IC) and visual cortex (VC) by magnetic resonance spectroscopy (MRS)] outcome measures were evaluated at baseline and at the 3-month follow-up. Side effects were monitored throughout the treatment course. Compared with the control group, the bumetanide group showed significant reduction in symptom severity, as indicated by both total CARS score and number of items assigned a score >= 3. The improvement in clinical symptoms was confirmed by CGI. GABA/Glx ratio in both the IC and VC decreased more rapidly over the 3-month period in the bumetanide group than that in the control group. This decrease in the IC was associated with the symptom improvement in the bumetanide group. Our study confirmed the clinical efficacy of bumetanide on alleviating the core symptoms of ASD in young children and it is the first demonstration that the improvement is associated with reduction in GABA/Glx ratios. This study suggests that the GABA/Glx ratio measured by MRS may provide a neuroimaging biomarker for assessing treatment efficacy for bumetanide.
Authors
Lingli Zhang, Chu-Chung Huang, Yuan Dai, Qiang Luo, Yiting Ji, Kai Wang, Shining Deng, Juehua Yu, Mingyu Xu, Xiujuan Du, Yun Tang, Chun Shen, Jianfeng Feng, Barbara J. Sahakian, Ching-Po Lin and Fei Li.
Acknowledgments
The authors thank Dr. Wei-Guang Li from Shanghai Jiao Tong University, Shanghai, China, for his insightful discussions on the possible molecular mechanisms of bumetanide. During the preparation of this manuscript, Dr Luo was a Visiting Fellow at Clare Hall, Cambridge, the UK. The work was financially supported by the Shanghai Municipal Commission of Health and Family Planning (No. 2017ZZ02026, No. 2018BR33, No. 2017EKHWYX-02 and No. GDEK201709), Shanghai Shenkang Hospital Development Center (No. 16CR2025B), Shanghai Municipal Education Commission (No. 20152234), National Natural Science Foundation of China (No. 81571031, No. 81761128035, No. 81930095, No. 81873909, No. 81222012, No. 912321023, No. 81701334 and No. 81703249), Shanghai Committee of Science and Technology (No. 17XD1403200, No. 17ZR1444400, No. 19410713500, No. 18DZ2313505), Xinhua Hospital of Shanghai Jiao Tong University School of Medicine (2018YJRC03, Talent introduction-014 and Top talent-201603), National Human Genetic Resources Sharing Service Platform (2005DKA21300), the National Key Research and Development Program of China (2018YFC0910503), 111 Project (No. B18015), the Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01), Guangdong Key Project in “Development of new tools for diagnosis and treatment of Autism” (2018B030335001), Taiwan Ministry of Science and Technology (MOST 108-2321-B-010-010-MY2), and ZJ Lab.
Return to top of page.
| |
|
Jan 28 2020 Fetal Timeline Maternal Timeline News
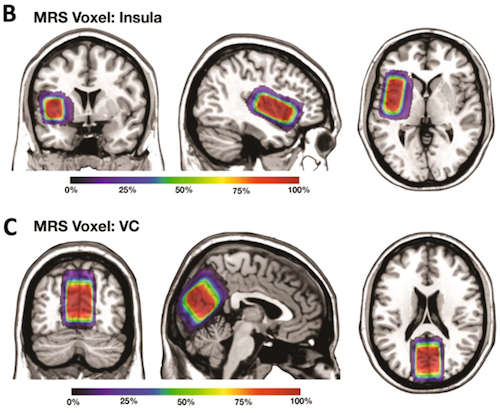 Among 102 participants recruited to this study, 81 participants completed the trial. These figures show the average voxel placement in (B) Left Insula and (C) Left Visual Cortex (VC) in all study participants that was transformed to the MNI standard space. The colormaps indicate the percentage of overlap. CREDIT All participating universities.
|



