|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
Developmental Biology - Sight Development Surprise in How Eyes 'See' Axons grow from the eye into various brain regions, including a visual processing area in the thalamus of the brain called the Lateral Geniculate Nucleus (LGN). Neuroscientists at the Fralin Biomedical Research Institute of the Virginia Tech Carilion Research Institute (VTC), recently published new research on this process in the journal: Proceedings of the National Academy of Sciences (PNAS). Their work reveals a surprising clue about our intricate visual processing system as it forms in-utero. Although previous studies had shown signals from retinal cells influence the LGN's cellular and molecular structure, this new research describes how retinal cells recruit star-shaped glial cells called astrocytes in order to promote inhibitory neuron growth into the LGN. Inhibitory neurons play crucial roles in refining visual processing, but how they form in the LGN is still not well understood. The research team, led by co-authors Michael Fox, Professor and Director of the Fralin Biomedical Research Institute, VTC Center for Neurobiology Research, and William Guido, Chair of the University of Louisville School of Medicine, Department of Anatomical Sciences and Neurobiology, asked a broad question: What happens to the LGN if retinal inputs never develop? Scientists used transcriptomic profiling, a technique to collect information from multiple mRNA transcripts in a biological sample, in order to compare changes in ordinary mice to mice without retinal connections. The research revealed mice without retinal connections had lower levels of an essential growth protein, called fibroblast growth factor 15 — a growth factor secreted by astrocytes. Without retinal inputs to the LGN area of their brains, there are stark changes in the make up of their LGN brain cells. Their LGN area is missing inhibitory interneurons, which ordinarily migrate over long distances into the LGN during fetal development. This reflects that without retinal inputs, astrocytes do not secrete growth protein — thus inhibiting interneurons from growing into the LGN. Previous studies described how astrocytes - the brain's most abundant cell type - have numerous functions. Not only do they regulate electrical impulses, but provide nutritional and immune support. "Astrocytes are a hot topic in neuroscience right now. And, although a lot of scientists are researching them, not many people have looked at how these cells are implicated in development of the visual thalamus." Significance Local inhibition, mediated by thalamic interneurons, contributes to the processing of visual information and is essential for vision. However, the mechanisms underlying the development of these interneurons remains unresolved. In this study, we sought to identify mechanisms that contribute to the long-distance migration of these interneurons into the visual thalamus. Our data show that innervation of the visual thalamus by retinal ganglion cells, the output neurons of the retina, is necessary for normal interneuron migration. Surprisingly, axons from these retinal ganglion cells appear to signal through astrocytes to contribute to thalamic interneuron migration. Astrocyte-derived cues may be a general mechanism for guiding interneuron migration throughout the brain. Abstract Inhibitory interneurons comprise a fraction of the total neurons in the visual thalamus but are essential for sharpening receptive field properties and improving contrast-gain of retinogeniculate transmission. During early development, these interneurons undergo long-range migration from germinal zones, a process regulated by the innervation of the visual thalamus by retinal ganglion cells. Here, using transcriptomic approaches, we identified a motogenic cue, fibroblast growth factor 15 (FGF15), whose expression in the visual thalamus is regulated by retinal input. Targeted deletion of functional FGF15 in mice led to a reduction in thalamic GABAergic interneurons similar to that observed in the absence of retinal input. This loss may be attributed, at least in part, to misrouting of interneurons into nonvisual thalamic nuclei. Unexpectedly, expression analysis revealed that FGF15 is generated by thalamic astrocytes and not retino-recipient neurons. Thus, these data show that retinal inputs signal through astrocytes to direct the long-range recruitment of interneurons into the visual thalamus. Authors Jianmin Su, Naomi E. Charalambakis, View ORCID ProfileUbadah Sabbagh, Rachana D. Somaiya, Aboozar Monavarfeshani, William Guido, and Michael A. Fox. Acknowledgments The authors thank Drs. S. Robel, S. Kliewer, and S. W. Wang for generously supplying Aldh1l1-GFP, Fgf15-/-, and Math5-/- mice, respectively; and Barbara O’Steen for her expert technical support. This work was supported by the National Institutes of Health Grants EY021222 (to M.A.F.), EY030568 (to M.A.F.), EY012716 (to W.G.), and NS113459 (to U.S.). Return to top of page. | Jan 29 2020 Fetal Timeline Maternal Timeline News 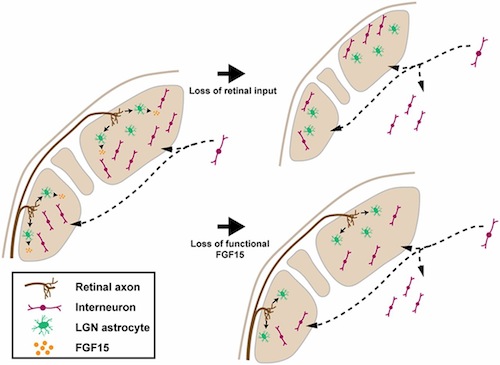 Retinal cells input signals from astrocytes assisted by fibroblast growth factor 15 — to recruit interneurons to enter the visual area of the brain'sthalamus. These schematics summarize the retinal input from astrocytes derived from FGF15 — along with the recruitment of GABA neurons into the dLGN and vLGN regions of the brain. CREDIT The Authors.
|
||||||||||||||||||||||||||||

