|

CLICK ON weeks 0 - 40 and follow along every 2 weeks of fetal development
|
||||||||||||||||||||||||||||
|
Developmental Biology - Alzheimer's Disease Possible Alzheimer's Breakthrough "This protein characteristically accumulates, or aggregates, within the center of plaque in AD patients, like the yolk of an egg — which is part of the reason we named it aggregatin. We're very excited as our study is likely the first systematic work combining the identification from a genome-wide association study of high dimensional brain-imaging data and experimental validation so perfectly in Alzheimer's disease." A research team led by Wang and Xiaofeng Zhu has filed for a patent through the Office of Research and Technology Management for novel Alzheimer's disease treatments and diagnosis based on this and related studies. Their research was published this month in the scientific journal Nature Communications and supported by grants from the National Institutes of Health (NIH) and the Alzheimer's Association. Genomic and brain imaging data was obtained from the Alzheimer's Disease Neuroimaging Initiative, which is supported by the NIH. Alzheimer's Disease affects millions Less understood is precisely how amyloid-beta actually leads to plaque formation — and where this new work appears to have broken new ground. Further, while there has been much research into what genes might influence whether or not someone gets Alzheimer's, there is less understanding of genes that might be linked to the progression of the disease, meaning the formation of plaque and subsequent atrophy of the brain. The Role of 'Aggregatin' Protein In the new work, researchers began by correlating roughly a million genetic markers (called Single-Nucleotide Polymorphisms, or SNPs) with brain images. They were able to identify a specific SNP in the FAM222 gene, which is linked to different patterns of regional brain atrophy. Further experiments suggested "aggregatin" is not only associated with AD patient-related beta-amyloid plaques and regional brain shrinkage, but attaches to amyloid beta peptide to facilitate plaque formation. When researchers injected mouse models with the "aggregatin" protein from the FAM222A gene, plaque (amyloid deposits) formation accelerated in the brain, resulting in more neuroinflammation and cognitive dysfunction. This happens because the protein binds directly to the amyloid beta peptide, facilitating aggregation and placque formation, says Wang. Conversely, when the protein is suppressed, plaques are reduced with neuroinflammation and cognitive impairment alleviated. These findings indicate that reducing levels of "aggregatin" protein and inhibiting its interaction with amyloid beta peptide could potentially be therapeutic — not necessarily to prevent Alzheimer's but to slow its progression. Abstract Alzheimer’s disease (AD) is characterized by amyloid plaques and progressive cerebral atrophy. Here, we report FAM222A as a putative brain atrophy susceptibility gene. Our cross-phenotype association analysis of imaging genetics indicates a potential link between FAM222A and AD-related regional brain atrophy. The protein encoded by FAM222A is predominantly expressed in the CNS and is increased in brains of patients with AD and in an AD mouse model. It accumulates within amyloid deposits, physically interacts with amyloid-Aß (Aß) via its N-terminal A? binding domain, and facilitates A? aggregation. Intracerebroventricular infusion or forced expression of this protein exacerbates neuroinflammation and cognitive dysfunction in an AD mouse model whereas ablation of this protein suppresses the formation of amyloid deposits, neuroinflammation and cognitive deficits in the AD mouse model. Our data support the pathological relevance of protein encoded by FAM222A in AD. Authors Tingxiang Yan, Jingjing Liang, Ju Gao, Luwen Wang, Hisashi Fujioka, The Alzheimer Disease Neuroimaging Initiative, Xiaofeng Zhu and Xinglong Wang. Acknowledgments This work is supported by grants from the US National Institutes of Health (RF1AG056320 to X.W.) and the US Alzheimer’s Association (AARG-17–499682 to X.W.). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12–2–0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Case Western Reserve University is one of the country's leading private research institutions. Located in Cleveland, we offer a unique combination of forward-thinking educational opportunities in an inspiring cultural setting. Our leading-edge faculty engage in teaching and research in a collaborative, hands-on environment. Our nationally recognized programs include arts and sciences, dental medicine, engineering, law, management, medicine, nursing and social work. About 5,100 undergraduate and 6,200 graduate students comprise our student body. Visit case.edu to see how Case Western Reserve thinks beyond the possible. Return to top of page. | Jan 30 2020 Fetal Timeline Maternal Timeline News 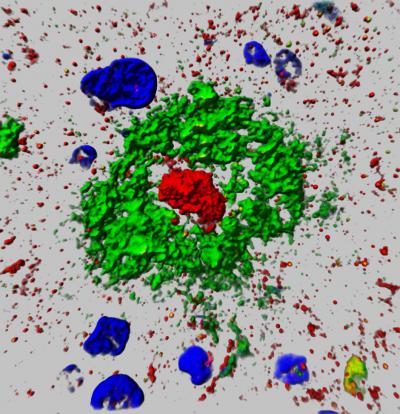 An image of the 'Aggregatin' protein from the paper published by Wang, Zhu and collaborators. CREDIT Case Western Reserve University
|
||||||||||||||||||||||||||||

