|
|
Developmental Biology - Opsins, the Light-Sensing Genes
Fat Cells Sense Sunlight
Lack of sunlight can lead to problems beyond seasonal affective disorder...
Yes, fat cells deep under your skin sense light. And when our bodies do not get enough exposure to the right kinds of light, fat cells behave differently.
This discovery, published Jan. 21, 2020, in the journal Cell Reports, was uncovered by scientists at Cincinnati Children's Hospital studying how mice control their body temperature. What they found has implications for beyond how mice stay warm.
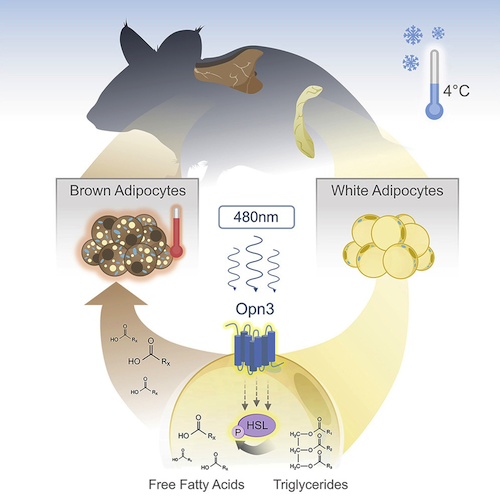
The study shows how light exposure regulates two kinds of fat cells to work together in producing raw materials needed by all cells to generate energy. The scientists believe disruption in this fundamental metabolic process reflects an unhealthy aspect of modern life — spending too much time indoors.

Expression of the OPN3 gene (seen as BLUE) in mouse white fat cells in two locations. 1. Upper neck to under forelimb where it connects to shoulder 2. Lower image shows white fat cells in groin fat depot.
CREDIT The Authors.
"Our bodies evolved over the years under the sun's light, including developing light-sensing genes called opsins," says Richard Lang, PhD, a developmental biologist and senior author of the study. "But now we live so much of our days under artificial light, which does not provide the full spectrum of light we all get from the sun."
Lang directs the Visual Systems Group at Cincinnati Children's and has authored or co-authored more than 120 research papers, including many related to eye development and how light interacts with cells beyond the eye.
"This paper represents a significant change in the way we view the effects of light on our bodies," adds Lang.
Shining new light on the role of light
Many people understand that certain wavelengths of light can be harmful, such as gamma radiation from a nuclear bomb or too much ultraviolent light from the sun burning our skin. This study from Lang and colleagues describes a different, healthy role for light exposure.
Despite the fur of a mouse, or the clothing of a person, light does get inside our bodies. Photons (the fundamental particles of light) may slow down and scatter around once they pass the outer layers of skin, Lang says. But they really do get in, and when they do, they affect how cells behave.
Lang's work in this direction dates back to 2013, when he led a study published in Nature, that demonstrated how light exposure affected eye development in fetal mice. More recently, in 2019, Lang and colleagues published two more papers, one in April in Nature Cell Biology that reported possible benefits of light therapy for eye development in preterm infants, and another study in October in Current Biology that details how light receptors in the skin help mice regulate their internal clocks.
The new study in Cell Reports includes important contributions from Russell Van Gelder MD, PhD, and Ethan Buhr PhD, from the University of Washington, and Randy Seeley PhD, University of Michigan.
"This idea of light penetration into deep tissue is very new, even to many of my scientific colleagues," Lang says. "But we and others have been finding opsins located in a variety of tissue types. This is still just the beginning of this work."
How light ignites an internal fire
In the latest findings, the research team studied how mice respond when exposed to chilly temperatures of about 40° F. They already knew that mice, much like humans, use both a shivering response and an internal fat-burning response to heat themselves.
Deaper analysis revealed in the absence of the OPN3 gene, internal heating is compromised. Specifically in response to a 480-nanometer wavelength of blue light. This wavelength is a natural part of sunlight but occurs only in low levels of most artificial light.
When the light exposure occurs, OPN3 prompts white fat cells to release fatty acids into the bloodstream. Various types of cells can use these fatty acids as energy to fuel their activities. But brown fat literally burns the fatty acids (in a process called oxidation) to generate heat that warms up the chilly mice.
When mice were bred without the OPN3 gene, they failed to warm up as much as other mice did when placed in chilly conditions. But surprisingly, even mice with the OPN3 gene failed to warm up when exposed to light without the blue wavelength.
This data prompted the team to conclude that sunlight is required for normal energy metabolism. At least in mice. While the scientists strongly suspect that a similar light-dependent metabolic pathway exists in humans, they need to complete another series of experiments to prove it.
"If the light-OPN3 adipocyte pathway exists in humans, there are potentially broad implications for human health," the study states. "Our modern lifestyle subjects us to unnatural lighting spectra, exposure to light at night, shift work, and jet lag, all of which result in metabolic disruption. Based on the current findings, it is possible that insufficient stimulation of the light-OPN3 adipocyte pathway is part of an explanation for the prevalence of metabolic deregulation in industrialized nations where unnatural lighting has become the norm."
What's next?
It likely will require several years of study to flesh out this discovery.
Someday, "light therapy" could become a method for preventing metabolic syndrome from developing into diabetes. Replacing indoor lights with better, full-spectrum lighting systems also could improve public health, Lang says.
However, more study is needed to pin down the potential therapeutic value of light therapy. Questions to answer include determining how much sunlight is needed to support a healthy metabolism and whether people battling obesity might lack a functional OPN3 gene in their fat cells. Also unknown: when would light therapy matter most: for pregnant mothers? For infants and children? Or for fully developed adults?
For now, "If people want to take anything personal away from this, you likely can't go wrong by spending more time outside!"
Richard Lang PhD
High Lights
• Adipocytes express encephalopsin (OPN3), a 480 nm blue-light-sensitive opsin
• Mice lacking OPN3 or blue light have diminished thermogenesis during cold exposure
• Loss of OPN3 reduces oxygen consumption and energy expenditure
• White adipocyte OPN3 promotes lipolysis during cold exposure
Summary
Almost all life forms can detect and decode light information for adaptive advantage. Examples include the visual system, in which photoreceptor signals are processed into virtual images, and the circadian system, in which light entrains a physiological clock. Here we describe a light response pathway in mice that employs encephalopsin (OPN3, a 480 nm, blue-light-responsive opsin) to regulate the function of adipocytes. Germline null and adipocyte-specific conditional null mice show a light- and Opn3-dependent deficit in thermogenesis and become hypothermic upon cold exposure. We show that stimulating mouse adipocytes with blue light enhances the lipolysis response and, in particular, phosphorylation of hormone-sensitive lipase. This response is Opn3 dependent. These data establish a key mechanism in which light-dependent, local regulation of the lipolysis response in white adipocytes regulates energy metabolism.
Authors
Gowri Nayak, Kevin X. Zhang, Shruti Vemaraju, Yoshinobu Odaka, Ethan D. Buhr, Amanda Holt-Jones, Stace Kernodle, April N. Smith, Brian A. Upton, Shane D’Souza, Jesse J. Zhan, Nicolás Diaz, Minh-Thanh Nguyen, Rajib Mukherjee, Shannon A. Gordon, Gang Wu, Robert Schmidt, Xue Mei, Nathan T. Petts, Matthew Batie, Sujata Rao, John B. Hogenesch, Takahisa Nakamura, Alison Sweeney, Randy J. Seeley, Russell N. Van Gelder, Joan Sanchez-Gurmaches and Richard A. Lang.
Acknowledgments
The authors thank Paul Speeg for excellent mouse colony management and the Transgenic Animal and Genome Editing Core at Cincinnati Children’s Hospital. We thank Kwoon Y. Wong at the University of Michigan, Ann Arbor, for providing the Opn4cre; Z/EG mouse line. This work was supported by NIH R01 GM124246 (to E.D.B.), R01EY026921 (to R.N.V.G.), P30EY001730 (to the University of Washington), R01 DK107530 (to T.N.), R01 EY027077 (to R.A.L. and S.R.), R01 EY027711 (to P. Michael Iuvone, Emory University , and R.A.L.); NIGMS T32 GM063483 (to the University of Cincinnati Medical Scientist Training Program); NIH P30 DK089503 (to R.J.S. and the Michigan Nutrition Obesity Research Center); the Mark J. Daily, MD Research Fund (to the University of Washington); and unrestricted grants to the University of Washington and Emory University Departments of Ophthalmology from Research to Prevent Blindness . This work was also supported by a Packard Foundation fellowship (to A.S.); American Heart Association grant 18CDA34080527 (to J.S.-G.); American Heart Association postdoctoral fellowship 19POST34380545 (to R.M.); and funds from the Goldman Chair of the Abrahamson Pediatric Eye Institute at Cincinnati Children’s Hospital Medical Center.
Tail Infrared Thermography (FLIR)
Adult Opn3fl/fl and Adipoq-cre; Opn3fl/fl animals were placed in a tubular mouse restraint (Kent Scientific, Torrington, CT). These restraints permitted respiration via a slotted nose cone but immobilized the animal while exposing the tail through a rear port. IR thermographic images were taken with a FLIR T530 infrared camera (FLIR® Systems, Wilsonville, OR). Tail temperatures were quantified by describing a pixel-averaged circular region of interest of consistent size and rostrocaudal distance from the base of the tail.
Quantification and Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 4.00 (GraphPad Software), Microsoft Excel and MATLAB 2018a (Figure 6). Two-tailed distribution, two-sample unequal variance t-test was used to determine the statistical significance between two independent groups. Time series datasets between two groups (Figures 4, 5, and 6) were analyzed via one-way repeated-measures ANOVA. Datasets involving two or more factors (Figures 6G–6I) were analyzed by 2-way ANOVAs with Holm-Šídák corrected multiple comparisons.
Data and Code Availability
Whole-genome expression profiles are available at accession number GEO: GSE140757.
About the study
This work was supported by grants from the National Institutes of Health, (R01GM124246, R01EY026921, P30EY001730, R01DK107530, R01EY027077, R01EY027711, R01EY004864, T32GM063483, P30 DK089503); the Mark J. Daily MD Research Fund; Research to Prevent Blindness; the Packard Foundation; the American Heart Association; and the Goldman Chair of the Abrahamson Pediatric Eye Institute at Cincinnati Children's Hospital Medical Center.
In addition to Lang, scientists from the University of Washington, University of Michigan, and Emory University contributed to this study. Co-authors from Cincinnati Children's and the University of Cincinnati include: Gowri Nayak, PhD, Kevin Zhang, BS, MS, Shruti Vemaraju, PhD, Yoshinobu Odaka, PhD, April Smith, MS, Brian Upton, BS, Shane D'Souza, BS, Minh-Thanh Nguyen, PhD, Rajib Mukherjee, PhD, Gang Wu, PhD, Robert Schmidt, BS, Xue Mei, PhD, Nathan Petts, BS, Matthew Batie, BA, John Hogenesch, PhD, Takahisa Nakamura, PhD, and Joan Sanchez-Gurmaches, PhD.
Return to top of page.
| |
|
Jan 31 2020 Fetal Timeline Maternal Timeline News
 Temperature changes in mouse white fat cells in two locations. 1. Upper neck to under forelimb where it connects to shoulder AND 2. White fat cells in groin lie above a layer of muscle and brown fat tissue that affect blood supply. CREDIT Cell Reports.
|




