|
|
Developmental Biology - Diagnosing Gene Abnormalities
New Prenatal Blood Test May Identify Gene Errors
A new study assessing DNA from live cells is "proof of concept" for potential non-invasive genetic diagnosis...
Non-Invasive Prenatal Tests (NIPTs) are currently used for fetal genetic disease screening in pregnant women, in contrast to invasive tests like amniocentesis which carries the risk of causing fetal harm. However, their results have not been consistently informative.
Now a report in The Journal of Molecular Diagnostics, describes the development of a new single-cell digital PCR assay without fixing, staining, whole-genome amplification or any other invasive method to assess DNA quality.
This noninvasive test enabled direct extraction of gene information from live fetal cells for evaluation, and may improve the likelihood of detecting fetal genetic anomalies.
Novel and noninvasive methods for definitively diagnosising fetal genetic abnormalities are much needed.
"Because NIPTs currently analyze small DNA fragments, it can be challenging to ascertain whether the origin of each DNA fragment is the mother or her fetus. We have observed that in some cases NIPT results are discordant with fetal genetic information that had been reported. This study serves as a proof of concept for noninvasive prenatal diagnosis using circulating fetal cells without any strict cell purification."
Kenichiro Hata MD PhD,
Department of Maternal-Fetal Biology, National Research Institute for Child Health and Development, Tokyo, Japan.
With this modified technique, up to 3,000 cells per well can be encapsulated in each droplet and simultaneously analyzed. The original sc-ddPCR system simultaneously assesses single-cell genetic information with high sensitivity and specificity. However, the original system is only useful for cell suspensions with a clear background (i.e., washed cell lines or clearly sorted cells with fluorescence-activated cell sorting). Researchers modified this sc-ddPCR system to improve the PCR environment in each droplet with higher sensitivity and specificity, which makes it possible to now assess single-cell genetic information from crudely purified nucleated cell samples with impurities.
To confirm the sensitivity of this modified sc-ddPCR system, investigators detected the genomic DNA of circulating male fetal cells in a crudely sorted cell suspension at the single-cell level derived from peripheral blood samples from mothers with male fetuses.
Investigators searched for the presence of the sex-determining region of the Y gene (SRY), which is responsible for initiating male sex determination.
Analysis of 13 blood samples indicated that only circulating fetal cells from the three pregnant women carrying male fetuses tested positive for the SRY gene. Cells from the 10 pregnant women carrying female fetuses did not.
This method confirmed the sensitivity of the sc-ddPCR system, basing it on the genomic DNA of circulating male fetal cells detected in a crude blood cell suspension taken from mothers only carrying male fetuses (at the single-cell level). Thus it serves as a proof of concept for noninvasive prenatal diagnosis.
"In the future, by optimizing cell sorting and encapsulation and generating a more effective PCR environment in each droplet, this modified sc-ddPCR system may be a breakthrough analysis method that can be applied to various research realms and possibly to clinical diagnostic testing."
Kenichiro Hata MD PhD
Abstract
Noninvasive testing techniques are often used for fetal diagnosis of genetic abnormalities but are limited by certain characteristics, including noninformative results. Thus, novel methods of noninvasive definitive diagnosis of fetal genetic abnormalities are needed. The aim of this study was to develop a single-cell DNA analysis method with high sensitivity and specificity that enables direct extraction of genetic information from live fetal cells in a crude mixture for simultaneous evaluation. Genomic DNA from circulating fetal CD45-CD14- cells, an extremely rare cell type, extracted from 10-mL samples of maternal peripheral blood, was extracted using a single-cell–based droplet digital (sc-dd) PCR system with a modified amount of polymerase. A hexachloro-6-carboxyfluorescein–labeled RPP30 probe was used as an internal control and a 6-carboxyfluorescein–labeled SRY probe as a target. The results indicated that no droplets generated with samples from pregnant women carrying female fetuses were positive for both probe signals, whereas droplets prepared with samples from pregnant women carrying male fetuses were positive for both probe signals. The latter was considered a direct assessment of genetic information from single circulating male fetal cells. Thus, the modified sc-ddPCR system allows the detection of genetic information from rare target cells in a crudely purified cell population. This research also serves as a proof of concept for noninvasive prenatal definitive diagnosis.
Authors
Taisuke Sato, Yuki Ito, Osamu Samura, Hiroaki Aoki, Toru Uchiyama, Aikou Okamoto, Kenichiro Hata and Kenichiro Hata.
Acknowledgments
The authors express their gratitude to the patients and their family members for their participation in this study. We thank Nobuyuki Watanabe and Tomoko Minegishi (National Research Institute for Child Health and Development, Tokyo, Japan) for their help in cell-sorting techniques.
Supported in part by the Ogyaa Donation Foundation of Japan Association of Obstetricians and Gynecologists (A.O.) and by National Center for Child Health and Development grant NCCHD 2019-A4 (K.H.).
Disclosures: None declared.
Return to top of page.
| |
|
Feb 10 2020 Fetal Timeline Maternal Timeline News
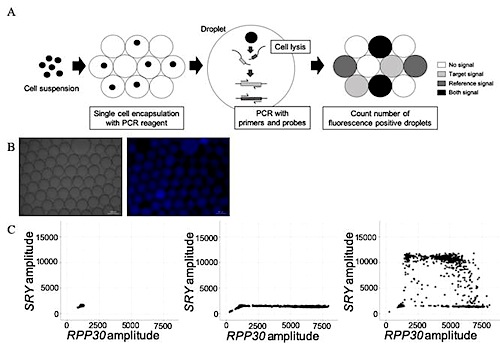 A) Schema of the single-cell-based droplet digital PCR (sc-ddPCR) system. Using the QX200 ddPCR system, each cell is simply encapsulated in one droplet. Up to 3,000 cells per well can be individually encapsulated. Subsequently, cell lysis and PCR with probes and primers are performed in each droplet. Finally, by quantifying the fluorescent droplets, single-cell genomic DNA is able to be assessed.
CREDIT Journal of Molecular Diagnostics
|



