|
|
Developmental Biology - Delayed Egg Implantation
How Do Some Mammals Pause Pregnancy?
A biochemical lag exists between conception and pregnancy in many mammals...
Some mammals postpone development of their embryos to await better conditions for these offspring. A recent study at the University of Washington (UW) Medicine Institute for Stem Cell and Regenerative Medicine, explored this reproductive mystery which occurs in more than 130 mammal species as well as in some marsupials.
The study was led by Abdiasis Hussein, a graduate student in the lab of Hannele Ruohola-Baker, professor of biochemistry and associate director of UW Medicine's Institute for Stem Cell and Regenerative Medicine. Their findings appear in Developmental Cell, a Cell Press scientific journal.
The results not only advance the understanding of delayed embryo implantation, but also suggest how some otherwise rapidly dividing cells — such as those in cancerous tumors — become inactive.
In this suspended state of pregnancy known as "embryonic diapause," an early-stage embryo refrains from implanting into the mother's uterus to be nourished and grow into a baby. Instead, like a seed, the embryo remains dormant until certain molecular regulators prod it to germinate.
Diapause, or delayed implantation, is a biological strategy for waiting out conditions that are unfavorable to sustaining newborn life, such as (1) lack of food, (2) insufficient maternal fat stores, or (3) older siblings who haven't been weaned.
Bears, armadillos, seals, some otters, badgers and other weasel-like animals undergo seasonal diapause as part of their reproductive cycle.
Many types of bears, for example, breed in the late spring or early summer. The female then voraciously hunts for food. Only when she has sufficient body fat and weight will one or more of her embryos implant months later - when she retreats to her den. Any cubs would then be born in late winter.
To learn what puts a biochemical hold-and-release on embryonic development, Abdiasis Hussein, Hannele Ruohola-Baker and their team induced diapause in a female mouse model by reducing the estrogen levels of those mice. Inducing diapause in mouse embryonic stem cells by starving those cells, researchers could then compare their response to actively growing mouse embryo stem cells in culture.
In the wild, some animal embryos will delay implantation until their mother has enough energy and nutrients in her body to support embryo bodies. Starvation or other stresses somehow provoke an embryo to stop time in an effort to protect its own survival.
Metabolism can be explained as the life-sustaining chemical activities cells carry out to convert substances into energy, build materials, and remove waste. By analyzing these reactions' end products — called metabolites — the scientists could begin to pull together a picture of what happens to cause diapause, and how cells are released from its clutches.
The scientists also looked at gene expression by comparing cell states to determine what might be influencing how DNA is interpreted. What critical proteins were being produced and in what amounts were suspended and/or in active states.
According to researcher Ruohola-Baker, epigenetic differences in interpreting the same DNA code, rather than alterations to DNA itself, may be key to understanding how embryos enter and exit diapause.
Further investigation pointed to a set of proteins vital to embryonic cell survival. The activity of the genes related to these proteins, as well as levels of certain amino acids, were ramped up in diapausal embryos. For example, by using CRISPR gene-editing technology, Hussein and Julie Mathieu, UW assistant professor of comparative medicine, squelched the flow of glutamine, an amino acid that controls an important metabolic (energy-use) pathway.
Researchers collected additional data indicating this and other metabolic factors which influence a catalytic enzyme called mTOR — regulates many cell processes, including cell proliferation, growth, and protein synthesis. mTOR is also involved in "sensing" cell nutrient and energy stores.
mTOR is already known as a central regulator of metabolism and physiology in mammalian aging and cancer. It also manages aspects of embryonic growth and development. In this study, situations that inhibited mTOR led to a distinct metabolic profile that characterizes diapause.
But, they also found this inhibition was reversible.
Understanding the mechanisms behind diapause could advance knowledge in human medicine and wildlife biology. Carol Ware, a UW professor of comparative medicine, believes that diapause is an essential means of survival for some species, while the result of environmental stress in others.
Research on the mechanism of diapause in animals is important to understanding if cellular response can be harnessed for clinical therapy, such as better in-vitro fertilization procedures to help people have children.
However, Hussein also believes this line of research might have significance in future cancer studies. Researchers may learn why and when cancer cells enter quiescence thus withstanding chemotherapy, only later to revive. Perhaps therapy eventually could be devised, he feels, which would 'wake up' cancer cells that coincided with the timing of anti-cancer drug therapies.
Highlights
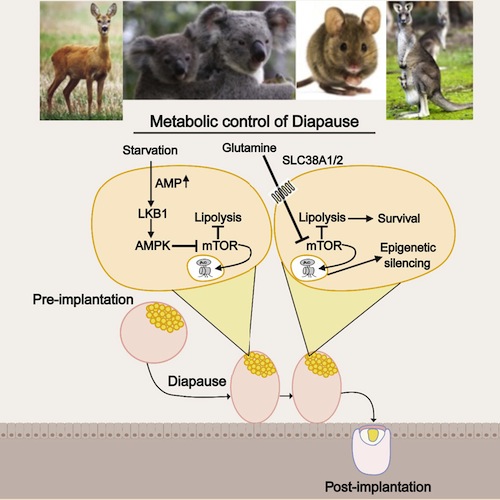
• Diapause is associated with increased lipolysis and glutamine transporter expression
• Upregulation of lipolysis in diapause is associated with downregulation of mTORC2
• Starvation results in a diapause-like state, enriched in glutamine transporters
• Inhibition of glutamine transporters leads to exit from the diapause epigenetic state
Summary
Regulation of embryonic diapause, dormancy that interrupts the tight connection between developmental stage and time, is still poorly understood. Here, we characterize the transcriptional and metabolite profiles of mouse diapause embryos and identify unique gene expression and metabolic signatures with activated lipolysis, glycolysis, and metabolic pathways regulated by AMPK. Lipolysis is increased due to mTORC2 repression, increasing fatty acids to support cell survival. We further show that starvation in pre-implantation ICM-derived mouse ESCs induces a reversible dormant state, transcriptionally mimicking the in vivo diapause stage. During starvation, Lkb1, an upstream kinase of AMPK, represses mTOR, which induces a reversible glycolytic and epigenetically H4K16Ac-negative, diapause-like state. Diapause furthermore activates expression of glutamine transporters SLC38A1/2. We show by genetic and small molecule inhibitors that glutamine transporters are essential for the H4K16Ac-negative, diapause state. These data suggest that mTORC1/2 inhibition, regulated by amino acid levels, is causal for diapause metabolism and epigenetic state.
Authors
Abdiasis M. Hussein, Yuliang Wang, Julie Mathieu, Lilyana Margaretha, Chaozhong Song, Daniel C. Jones, Christopher Cavanaugh, Jason W. Miklas, Elisabeth Mahen, Megan R. Showalter, Walter L. Ruzzo, Oliver Fiehn, Carol B. Ware, C. Anthony Blau and Hannele Ruohola-Baker.
Return to top of page.
| |
|
Feb 12 2020 Fetal Timeline Maternal Timeline News
 Seals and other pinnepeds are among the mammals whose early-stage embryos can enter diapause - a temporary dormant state - and then implant to develop later. The timing of pregnancy and birth are thereby postponed to occur when conditions are more favorable for survival. CREDIT Alice C. Gray
|



