|
|
Developmental Biology - Leukaemia
Rogue Cell Communication Leads to Leukaemia
Deciphering how rogue communication between blood stem cells cause leukaemia....
Blood cancers, like leukaemia, occur when mutations in how stem cells "talk" to each other, leads to the production of too many blood cells. This new discovery could pave the way for treatments to block such mutations. The work was just published in the journal Science.
Researchers represented an international collaboration between the Universities of York and Dundee (United Kingdom), Osnabruck (Germany), Helsinski (Finland), Stanford and New York Universities (USA). Together the team discovered blood cell surface mutations create deviations from normal cell communication, prompting blood cell growth to spiral out of control.
Using super-resolution fluorescent microscopy in real time, researchers observed how blood stem cells receive instructions via proteins called cytokines, the largest and most diverse family of signalling proteins, and critical in the development of blood cells and the immune system.
Understanding this process led researchers to discover mutations associated with certain types of blood cancers can cause blood stem cells to 'go rogue' and communicate without cytokines.
Stem cells that transmit uncontrolled signals cause normal blood cell development to become overrun, creating an imbalance in healthy white and red blood cells and platelets.
"Our bodies produce billions of blood cells every day via cytokine signalling. Cytokines act like a factory supervisor, tightly regulating blood cells and controlling the development and proliferation of different blood cell types.
"Our observations led us to a previously unknown mechanism — individual mutations trigger blood stem cells to start signalling independently of cytokines...causing the normal system to become out of control and lead to diseases like leukaemia. Understanding this mechanism may enable identification of targets for development of new drugs."
Ian Hitchcock PhD, Professor, York Biomedical Research Institute and Department of Biology, University of York.
For the first time, researchers used a combination of molecular modelling, structural biology, biophysics, super-resolution microscopy and cell biology to demonstrate which specific receptors (on the surface of blood stem cells) are linked by cytokines to form pairs.
"By directly visualising individual receptors under the microscope, we were able to resolve a controversy that has preoccupied the field for more than 20 years."
Jacob Piehler PhD, Professor, Osnabrück University, and co-author of the study.
Activated by Interaction
Cytokines trigger immune responses when they bind to cell surface receptors that recognise them. Class I cytokine receptors rely on the associated Janus kinase 2 (JAK2) to initiate signal transduction. Debate continues over whether activation involves ligand induced dimerization of these receptors or ligand induced conformational change of preformed dimers.
Wilmes et al. imaged cytokine receptors in the plasma membranes of live human cells by single-molecule fluorescence microscopy and observed ligand-induced dimerization.
They found that the JAK2 pseudokinase domains contribute to dimerization and that hyperactive JAK2 mutants promote dimerization, consistent with the model that dimerization triggers activation.
Abstract
Homodimeric class I cytokine receptors are assumed to exist as preformed dimers that are activated by ligand-induced conformational changes. We quantified the dimerization of three prototypic class I cytokine receptors in the plasma membrane of living cells by single-molecule fluorescence microscopy. Spatial and spatiotemporal correlation of individual receptor subunits showed ligand-induced dimerization and revealed that the associated Janus kinase 2 (JAK2) dimerizes through its pseudokinase domain. Oncogenic receptor and hyperactive JAK2 mutants promoted ligand-independent dimerization, highlighting the formation of receptor dimers as the switch responsible for signal activation. Atomistic modeling and molecular dynamics simulations based on a detailed energetic analysis of the interactions involved in dimerization yielded a mechanistic blueprint for homodimeric class I cytokine receptor activation and its dysregulation by individual mutations.
Authors
Stephan Wilmes, Maximillian Hafer, Joni Vuorio, Julie A. Tucker, Hauke Winkelmann, Sara Löchte, Tess A. Stanly, Katiuska D. Pulgar Prieto, Chetan Poojari, Vivek Sharma, Christian P. Richter, Rainer Kurre, Stevan R. Hubbard, K. Christopher Garcia, Ignacio Moraga, Ilpo Vattulainen, Ian S. Hitchcock and Jacob Piehler.
Return to top of page.
| |
|
Feb 13 2020 Fetal Timeline Maternal Timeline News
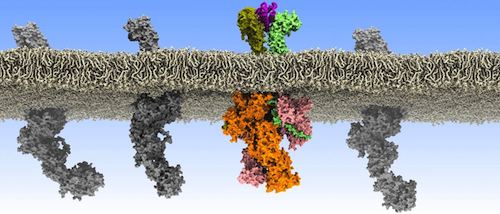 Rogue communications in the membrane of blood stem cells. CREDIT Ilpo Vattulainen and Joni Vuorio from the University of Helsinki.
|



