|
|
Developmental Biology - Fetal Stem Cell Transplants
Treating Fetal Disorders With Stem Cell Transplants
Stem cells can migrate to the fetal brain and other body locations and help produce crucial missing proteins...
Administering stem cell or enzyme therapy in utero may be a path to alleviate congenital diseases often resulting in pregnancy loss. This is according to a new study by University of California San Francisco (UCSF) researchers. Their work, shows that stem cells can enter the fetal brain during prenatal development and make up for cells that fail to produce an essential protein.
Each year, about 24,000 women in the US lose a pregnancy. One of the major contributors to this problem is a group of congenital diseases that can cause a condition called hydrops, in which fluid accumulates in the fetus, often fatally.
"This group of vulnerable patients has been relatively ignored in the fetal surgery world. We know they could potentially benefit from a number of medical therapies — this is our first foray into treating one of those diseases."
Tippi MacKenzie MD, senior author.
Tippi MacKenzie MD, has worked for a decade at UCSF developing novel therapies to treat inherited diseases. Her latest study published in Science Translational Medicine explored treatments for MPS7, also known as Sly syndrome, a disorder caused by a mutation within a single gene. Patients with Sly syndrome lack an enzyme to process large chains of sugar molecules. The number of incidents of MPS7 is difficult to determine, as it is believed many fetuses with the disorder die before birth. Those that survive must receive regular injections of the missing enzyme.
But as the so-called blood-brain barrier protects the brain from potentially damaging substances in blood - is not fully developed before birth — MacKenzie believes the enzyme could successfully enter the fetal brain. However, after birth, clinicians must infuse the missing enzyme directly into the brain.
Repeated enzyme infusions for metabolic disorders can cause patients to develop an immune response to the enzyme, as their bodies view the enzyme as "foreign." Infusing it before birth could prevent this immune response, while the fetal immune system views new proteins as "friendly," and does not reject them.
MacKenzie co-directs the UCSF Center for Maternal-Fetal Precision Medicine. She is among the first physicians to attempt enzyme replacement during fetal development. Led by co-first author Russell Witt MD, these experiments with mice have proved successful, with researchers discovering that in utero treatment with the missing enzyme dramatically improved survival of pups to birth.
Researchers continued to administer the enzyme after birth, ultimately seeing immune tolerance in the enzyme and improvements in multiple organs, including brain and liver. Comprehensive testing of the mice conducted in collaboration with the UCSF lab of Saul Villeda PhD, recorded significantly improved strength in the grip of MPS7 mice - which is generally weak. In some cases their grip strength approached that of normal mice.
All told, the research team found that in utero enzyme replacement conferred three major improvements. MacKenzie: "It will enter the brain, the mice develop immune tolerance for it, and the treatment helps sustain the fetus through birth."
These combined advantages, she said, overcome the main limitations of the current approach of delivering enzyme treatment after birth — but not all of them. Even if the treatment were successfully delivered in the womb, challenges would still arise. Because the enzyme only lasts about two weeks in the body, patients would still require regular injections after birth. And at that point, the blood-brain barrier is fully developed, so the enzyme can't cross into the brain.
Ultimately, MacKenzie says the answer may lie in giving fetuses stem cells, which could differentiate into new cells - in both the brain and the rest of the body - that could produce the enzyme that the faulty ones don't.
To explore this possibility, MacKenzie worked with research fellow and surgery resident Quoc-Hung Nguyen MD, to transplant blood-forming stem cells from normally developing mice into fetal mice carrying a genetic mutation that causes MPS7. The researchers were most interested to see whether these cells could reach the brain, and whether they would change into cells called microglia, immune cells that originate from blood-forming stem cells. In a normally developing fetus, once matured, microglia produce and store the necessary enzyme, as well as regulate the immune environment of the brain.
Others have tried transplanting these stem cells, but usually after birth, said Nguyen, and it has been challenging to obtain fully functioning microglia. "We wanted to test this transplant before birth, using the environment of the fetal brain where, in a normal fetus, stem cells are migrating into the brain to become microglia," he said. "One of our big findings is that these cells truly do become microglia, so there's a huge advantage to transplanting them before birth."
Nguyen tagged the transplanted cells with a fluorescent marker so he could easily identify them and verify that they had successfully crossed the blood-brain barrier and migrated into the brain. To confirm that the transplanted cells were acting as functional microglia, Nyugen sequenced the RNA that the cells were producing and saw that it matched the proper signature of protein production by microglia.
He also confirmed that the stem cells had also made their way to the liver, kidney, and other organs, and that they became the appropriate cell type needed to produce the needed enzyme in those organs.
The researchers saw that once the stem cells had engrafted in the brain and body and differentiated, they were able to deliver the enzyme to nearby cells and restore their function, a process called cross-correction. The transplanted cells thus fended off the liver disease and other complications associated with MPS7 for the lifespan of the animals.
"We found that even if you have only one or two percent healthy cells circulating, you can drastically improve, for example, liver disease," said MacKenzie. "One good cell can, in effect, correct multiple other cells."
Another advantage of the proposed treatments is that, in humans, they would be performed using the same techniques now used for fetal blood transfusions, which have been performed for decades in hospitals all around the country.
"That means that if we do get to a stage of performing fetal therapy for these diseases in humans, the treatments could someday be offered at multiple centers around the world, and the family doesn't necessarily have to travel a long distance to get the care they need," said MacKenzie.
There are a large number of other heritable metabolic disorders that arise from similar faulty single genes, and Nyugen and MacKenzie agree that their approach may be a useful one for these conditions as well.
"These exciting findings are just the tip of the iceberg," said Nyugen. "They open up a whole new approach to treating a range of diseases. At the same time, there's also a lot of work to do to optimize the treatment for humans."
MacKenzie is applying to the U.S. Food and Drug Administration to launch a clinical trial of enzyme replacement therapy that will ultimately enroll 10 patients with MPS7 and related metabolic disorders.
A similar trial is already underway, transplanting mothers' stem cells into developing fetuses to treat a blood disorder called alpha thalassemia. With the proposed trial of enzyme replacement therapy, MacKenzie hopes to expand the scope of fetal molecular therapies and treat metabolic disorders as well as blood disorders in humans.
While fetal molecular therapies are not yet common, MacKenzie points out that the environment and resources at UCSF allow her team to broaden the field, even as it is developing. She has had successful deliveries following in utero stem cell transplant treating alpha thalassemia.
"With this work, we're pushing the frontiers of fetal surgery into newer, less invasive therapies. At the same time, colleagues at UCSF are performing genetic sequencing of fetuses with hydrops, so that one day we can identify and treat these genetic conditions before birth. It's exactly this sort of clinical and research environment that can accelerate moving a therapy like this one into humans."
Tippi MacKensie MD
Abstract
When treating at birth is too late
Mucopolysaccharidosis type VII (MPS7) is a rare and severe lysosomal storage disorder, which causes dysfunction of multiple organs including the brain and may be associated with undiagnosed cases of fetal death. By the time of birth, the organ damage may already be severe and the fetus may not survive at all. Thus, the prenatal period provides the most promising opportunity for intervention. Nguyen et al. assessed two prenatal approaches, in utero enzyme replacement therapy and in utero hematopoietic stem cell transplantation, and demonstrated the potential of these treatments to improve survival and functional outcomes in a mouse model of MPS7.
Abstract
Mucopolysaccharidosis type VII (MPS7) is a lysosomal storage disorder (LSD) resulting from mutations in the B-glucuronidase gene, leading to multiorgan dysfunction and fetal demise. While postnatal enzyme replacement therapy (ERT) and hematopoietic stem cell transplantation have resulted in some phenotypic improvements, prenatal treatment might take advantage of a unique developmental window to penetrate the blood-brain barrier or induce tolerance to the missing protein, addressing two important shortcomings of postnatal therapy for multiple LSDs. We performed in utero ERT (IUERT) at E14.5 in MPS7 mice and improved survival of affected mice to birth. IUERT penetrated brain microglia, whereas postnatal administration did not, and neurological testing (after IUERT plus postnatal administration) showed decreased microglial inflammation and improved grip strength in treated mice. IUERT prevented antienzyme antibody development even after multiple repeated postnatal challenges. To test a more durable treatment strategy, we performed in utero hematopoietic stem cell transplantation (IUHCT) using congenic CX3C chemokine receptor 1–green fluorescent protein (CX3CR1-GFP) mice as donors, such that donor-derived microglia are identified by GFP expression. In wild-type recipients, hematopoietic chimerism resulted in microglial engraftment throughout the brain without irradiation or conditioning; the transcriptomes of donor and host microglia were similar. IUHCT in MPS7 mice enabled cross-correction of liver Kupffer cells and improved phenotype in multiple tissues. Engrafted microglia were seen in chimeric mice, with decreased inflammation near donor microglia. These results suggest that fetal therapy with IUERT and/or IUHCT could overcome the shortcomings of current treatment strategies to improve phenotype in MPS7 and other LSDs.
Authors
Quoc-Hung Nguyen, Russell G. Witt and Saul Villeda, UCSF authors incuded Bowen Wang, Carlo Eikani, Jeremy Shea PhD, Lucas Smith PhD, Renan Sper PhD, and John D. MacKenzie MD. Joined by Gabrielle Boyle and Jaclyn Cadaoas of Ultragenyx, of Novato, Calif.
Acknowledgements
Funding: This study was supported by a grant from Ultragenyx to Tippi MacKenzie, funds from the UCSF Center for Maternal-Fetal Precision Medicine, a CIRM New Faculty grant to Tippi MacKenzie (RN3-06532), and NIH grant support (5T32HD007263-35) to Quoc-Hung Nguyen.
Disclosures: Co-authors Gabrielle Boyle and Jaclyn Cadaoas are employees of Ultragenyx.
About UCSF
The University of California, San Francisco (UCSF) is exclusively focused on the health sciences and is dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. UCSF Health, which serves as UCSF's primary academic medical center, includes top-ranked specialty hospitals and other clinical programs, and has affiliations throughout the Bay Area. Learn more at https://www.ucsf.edu, or see our Fact Sheet.
About ISGlobal
The Barcelona Institute for Global Health, ISGlobal, is the fruit of an innovative alliance between "la Caixa" and academic and government institutions to contribute to the efforts undertaken by the international community to address the challenges in global health. ISGlobal is a consolidated hub of excellence in research that has grown out of work first started in the world of health care by the Hospital Clínic and the Parc de Salut MAR and in the academic sphere by the University of Barcelona and Pompeu Fabra University. The pivotal mechanism of its work model is the transfer of knowledge generated by scientific research to practice, a task undertaken by the institute's Education and Policy and Global Development departments. ISGlobal has been named a Severo Ochoa Centre of Excellence and is a member of the CERCA programme of the Generalitat de Catalunya.
Return to top of page.
| |
|
Mar 3 2020 Fetal Timeline Maternal Timeline News
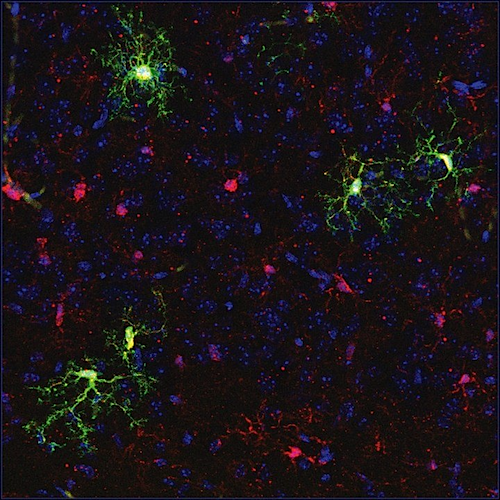 Stem cells transplanted in utero (GREEN) engrafted into fetal mouse brain tissue. CREDIT Nguyen/MacKenzie lab.
|



