|
|
Developmental Biology - Brain Gene DDX3X
Dozens of Mutations in Crucial Brain Gene
Numerous malfunctions in one brain growth gene now considered a developmental disability syndrome...
An international team of researchers — after pooling genetic samples from developmentally disabled patients around the world — identified dozens of new mutations in a single gene that appear to be critical to brain development.
"This is important as there are a handful of genes recognized as 'hot spots' for mutations causing neurodevelopmental disorders. The gene DDX3X is now going to be added to that list."
Debra Silver PhD, lead author and Aassociate Professor, Molecular Genetics and Microbiology, Duke School of Medicine, Raleigh, North Carolina, USA.
An analysis led by the Elliott Sherr lab at the University of California-San Francisco found that half of the DDX3X mutations in the 107 children studied caused a loss of function that made the gene stop working altogether, but the other half caused changes predicted to disrupt the function of the gene.
The DDX3X gene is carried by the X chromosome, which occurs twice in females, but only once in males. Only three of the children in the study were male, indicating an aberrant copy of the gene is probably lethal for males with their single X.
In humans, this syndrome often results in smaller brains and intellectual disability. Understanding how and why DDX3X mutations lead to developmental delay gives insight into how the gene should work.
With the finding that DDX3X was a common element in the developmental disabilities of these children, Silver's team "used a set of experimental tricks to see how it would lead to disease."
In mice, her team manipulated levels of the gene to see how cerebral cortex development is altered. She saw that changing levels of the gene led to fewer neurons being produced - depending on dose.
In most severe cases, Sherr's team found functional changes in DDX3X resulted in smaller or completely missing corpus collosum, the broad communications structure between the two halves of our brain.
In some cases, identical genetic spelling errors that occurred in several children led to polymicrogyria, an abnormal folding pattern on the surface of the brain.
Silver: "Not every mutation acts the same."
The collaborative team also tested how 'missense' mutations, where the protein is made but for some reason is defective, impairs brain development. In the most severe missense mutations, protein was formed into 'clumps' of RNA-protein inside neural stem cells — similar to protein clumps found in Alzheimer's disease, Silver explained.
Together, these issues point to a role for DDX3X in the genesis of developing neurons as the brain grows. Silver: "The way neurons are made and organized is disrupted. We know that this gene is required for early brain development which can cause a whole host of developmental problems."
Almost all of the mutations seen in children in the study were 'de novo,' meaning mutations happened during a child's early development, and were not inherited from a parent.
Parents of the children with these mutations have established the DDX3X Foundation to pursue better understanding of what causes the disease, identify therapies, and provide a supportive community for families.
Abstract Highlights
• Discovery of 107 mutations in the RNA helicase DDX3X causing cortical malformations
• Clinical severity is linked to reduced helicase activity and RNA-protein granules
• Ddx3x is required in neural progenitors to produce cortical neurons during development
• Severe missense mutations cause polymicrogyria and impair translation of targets
Summary
De novo germline mutations in the RNA helicase DDX3X account for 1%–3% of unexplained intellectual disability (ID) cases in females and are associated with autism, brain malformations, and epilepsy. Yet, the developmental and molecular mechanisms by which DDX3X mutations impair brain function are unknown. Here, we use human and mouse genetics and cell biological and biochemical approaches to elucidate mechanisms by which pathogenic DDX3X variants disrupt brain development. We report the largest clinical cohort to date with DDX3X mutations (n = 107), demonstrating a striking correlation between recurrent dominant missense mutations, polymicrogyria, and the most severe clinical outcomes. We show that Ddx3x controls cortical development by regulating neuron generation. Severe DDX3X missense mutations profoundly disrupt RNA helicase activity, induce ectopic RNA-protein granules in neural progenitors and neurons, and impair translation. Together, these results uncover key mechanisms underlying DDX3X syndrome and highlight aberrant RNA metabolism in the pathogenesis of neurodevelopmental disease.
Authors
Ashley L. Lennox, Mariah L. Hoye, Ruiji Jiang, Bethany L. Johnson-Kerner, Lindsey A. Suit, Srivats Venkataramanan, Charles J. Sheehan, Fernando C. Alsina, Brieana Fregeau, Kimberly A. Aldinger, Ching Moey, Iryna Lobach, Alexandra Afenjar, Dusica Babovic-Vuksanovic, Stéphane Bézieau, Patrick R. Blackburn, Jens Bunt, Lydie Burglen, Philippe M. Campeau, Perrine Charles, Brian H.Y. Chung,
Benjamin Cogné, Cynthia Curry, Maria Daniela D’Agostino, Nataliya Di Donato, Laurence Faivre,
Delphine Héron, A. Micheil Innes, Bertrand Isidor, Boris Keren, Amy Kimball, Eric W. Klee, Paul Kuentz,
Sébastien Küry, Dominique Martin-Coignard, Ghayda Mirzaa, Cyril Mignot, Noriko Miyake, Naomichi Matsumoto, Atsushi Fujita, Caroline Nava, Mathilde Nizon, Diana Rodriguez, Lot Snijders Blok, Christel Thauvin-Robinet, Julien Thevenon, Marie Vincent, Alban Ziegler, William Dobyns, Linda J. Richards, A. James Barkovich, Stephen N. Floor, Debra L. Silver, and Elliott H. Sherr.
Acknowledgements
This work was supported by the U.S. National Institutes of Health (R01NS083897, R21NS098176, R01NS110388, F31NS0933762, F32NS112566, R25, 1R01NS058721, 5R01NS050375, DP2GM132932); Australia National Health and Medical Research Council, (GNT1126153, GNT1120615); California Tobacco-Related Disease Research Grants Program 27KT-0003; HUGODIMS consortium (RC14_0107); Dandy-Walker Alliance; DDX3X Foundation; Duke Regeneration Next Initiative; French Ministry of Health and the Health Regional Agency from Poitou-Charentes; and Frédérique Allaire from the Health Regional Agency of Poitou-Charentes; Holland-Trice Foundation; Sandler Foundation; UCSF Program for Breakthrough Biomedical Research.
Return to top of page.
| |
|
Mar 5 2020 Fetal Timeline Maternal Timeline News
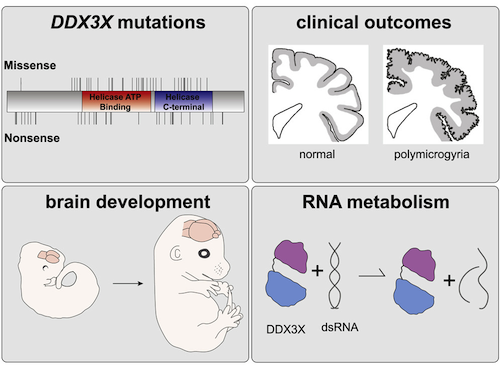 DDX3X gene mutations show it controls cortical development, regulating generation of neurons. Severe DDX3X mutations profoundly disrupt RNA helicase activity, inducing ectopic RNA- protein granules in neural progenitor cells as well as in neurons. CREDIT Duke University.
|



