|
|
Developmental Biology - COVID 19
A Potential Vaccine Against SARS-CoV-2
When tested in mice, the vaccine produces antibodies specific to SARS-CoV-2 at quantities thought to be sufficient for neutralizing the virus...
April 2, 2020 – University of Pittsburgh School of Medicine (UPMC) scientists have announced a potential vaccine against SARS-CoV-2, the new coronavirus causing the COVID-19 pandemic. When tested in mice, the vaccine, delivered through a fingertip-sized patch, produces antibodies specific to SARS-CoV-2 at quantities thought to be sufficient for neutralizing the virus.
The paper appeared today in EBioMedicine, which is published by The Lancet, and is the first study to be published after critique from fellow scientists at outside institutions that describes a candidate vaccine for COVID-19. The researchers were able to act quickly because they had already laid the groundwork during earlier coronavirus epidemics.
“We had previous experience on SARS-CoV in 2003 and MERS-CoV in 2014. These two viruses, which are closely related to SARS-CoV-2, teach us that a particular protein, called a spike protein, is important for inducing immunity against the virus. We knew exactly where to fight this new virus. That’s why it’s important to fund vaccine research. You never know where the next pandemic will come from.”
Andrea Gambotto MD, Associate Professor of Surgery, Pitt School of Medicine.
“Our ability to rapidly develop this vaccine was a result of scientists with expertise in diverse areas of research working together with a common goal.”
Louis Falo MD PhD, Professor and Chair of Dermatology, Pitt’s School of Medicine and UPMC - and co-senior author.
Compared to the experimental mRNA vaccine candidate that just entered clinical trials, the vaccine described in this paper — which the authors are calling PittCo Vacc, short for Pittsburgh Coronavirus Vaccine — follows a more established approach, using lab-made pieces of viral protein to build immunity. It works in the same way as the current flu shot.
Researchers also used a novel approach to deliver the drug, called a microneedle array, to increase potency. This array is a fingertip-sized patch of 400 tiny needles that delivers the spike protein pieces into the skin, where the immune reaction is strongest. The patch goes on like a Band-Aid and then the needles — which are made entirely of sugar mixed with protein pieces — simply dissolve into the skin.
“We developed this to build on the original scratch method used to deliver the smallpox vaccine to skin, but as a high-tech version that is more efficient and reproducible patient to patient,” explains Falo. “And it’s actually pretty painless — it feels kind of like Velcro.”
The system also is highly scalable. The protein pieces are manufactured by a “cell factory” — layers upon layers of cultured cells engineered to express the SARS-CoV-2 spike protein — that can be stacked further to multiply yield. Purifying the protein also can be done at industrial scale. Mass-producing the microneedle array involves spinning down the protein-sugar mixture into a mold using a centrifuge. Once manufactured, the vaccine can sit at room temperature until it’s needed, eliminating the need for refrigeration during transport or storage.
“For most vaccines, you don’t need to address scalability to begin with,” Gambotto explains. “But when you try to develop a vaccine quickly against a pandemic that’s the first requirement.”
When tested in mice, PittCo Vacc generated a surge of antibodies against SARS-CoV-2 within two weeks of the microneedle prick.
Those animals haven’t been tracked long term yet, but researchers point out that mice who got their MERS-CoV vaccine produced a sufficient level of antibodies to neutralize the virus for at least a year, and so far the antibody levels of the SARS-CoV-2 vaccinated animals seem to be trending the same.
Importantly, the SARS-CoV-2 microneedle vaccine maintains its potency even after being thoroughly sterilized with gamma radiation — a key step toward making a product that’s suitable for use in humans.
The authors are now in the process of applying for an investigational new drug approval from the U.S. Food and Drug Administration in anticipation of starting a phase I human clinical trial in the next few months.
“Testing in patients would typically require at least a year and probably longer. This particular situation is different from anything we’ve ever seen, so we don’t know how long the clinical development process will take. Recently announced revisions to the normal processes suggest we may be able to advance this faster.”
Louis Falo MD PhD, Professor and Chair of Dermatology, Pitt’s School of Medicine and UPMC.
When the embargo lifts, UPMC and Pitt will conduct a virtual press conference where the authors will answer questions from reporters.
Abstract
Background: CCoronaviruses pose a serious threat to global health as evidenced by Severe Acute Respiratory Syndrome
(SARS), Middle East Respiratory Syndrome (MERS), and COVID-19. SARS Coronavirus (SARS-CoV), MERS Coronavirus (MERS-CoV), and the novel coronavirus, previously dubbed 2019-nCoV, and now officially named SARS-CoV-2, are the causative agents of the SARS, MERS, and COVID-19 disease outbreaks, respectively. Safe vaccines that rapidly induce potent and long-lasting virus-specific immune responses against these infectious agents are urgently needed. The coronavirus spike (S) protein, a characteristic structural component of the viral envelope, is considered a key target for vaccines for the prevention of coronavirus infection.Methods: We first generated codon optimized MERS-S1 subunit vaccines fused with a foldon trimerization
domain to mimic the native viral structure. In variant constructs, we engineered immune stimulants (RS09 or flagellin, as TLR4 or TLR5 agonists, respectively) into this trimeric design. We comprehensively tested the pre-clinical immunogenicity of MERS-CoV vaccines in mice when delivered subcutaneously by traditional needle injection, or intracutaneously by dissolving microneedle arrays (MNAs) by evaluating virus specific IgG antibodies in the serum of vaccinated mice by ELISA and using virus neutralization assays. Driven by the urgent need for COVID-19 vaccines, we utilized this strategy to rapidly develop MNA SARS-CoV-2 subunit vaccines and tested their pre-clinical immunogenicity in vivo by exploiting our substantial experience with MNA MERS-CoV vaccines.
Findings: Here we describe the development of MNA delivered MERS-CoV vaccines and their pre-clinical immunogenicity. Specifically, MNA delivered MERS-S1 subunit vaccines elicited strong and long-lasting antigen-specific antibody responses. Building on our ongoing efforts to develop MERS-CoV vaccines, promising immunogenicity of MNA-delivered MERS-CoV vaccines, and our experience with MNA fabrication and delivery, including clinical trials, we rapidly designed and produced clinically-translatable MNA SARS-CoV-2 subunit vaccines within 4 weeks of the identification of the SARS-CoV-2 S1 sequence. Most importantly, these MNA delivered SARS-CoV-2 S1 subunit vaccines elicited potent antigen-specific antibody responses that were evident beginning 2 weeks after immunization. Interpretation: MNA delivery of coronaviruses-S1 subunit vaccines is a promising immunization strategy against coronavirus infection. Progressive scientific and technological efforts enable quicker responses to emerging pandemics. Our ongoing efforts to develop MNA-MERS-S1 subunit vaccines enabled us to rapidly design and produce MNA SARS-CoV-2 subunit vaccines capable of inducing potent virus-specific antibody responses. Collectively, our results support the clinical development of MNA delivered recombinant protein subunit vaccines against SARS, MERS, COVID-19, and other emerging infectious diseases. © 2020 The Author(s). Published by Elsevier B.V. This is an open access article under the CC BY license
Authors
Eun Kim, Geza Erdos, Shaohua Huang, Thomas W. Kenniston, Stephen C. Balmert, Cara Donahue Carey, V. Stalin Raj, Michael W. Epperly, William B. Klimstra, Bart L. Haagmans, Emrullah Korkmaz, Louis D. Falo Jr. and Andrea Gambotto.
Acknowledgments
AG is funded by NIH NIAID (R21-AI114264) and LDF is funded by NIH NIAMS (R01-AR074285, R01-AR071277, and R01-AR068249 to LDF). SCB is supported by a fellowship from the NIH National Cancer Institute (T32-CA175294). These funding institutions had no role in the study design, data collection, data analysis, and interpretation of this publication.
Return to top of page.
| |
|
Apr 6 2020 Fetal Timeline Maternal Timeline News
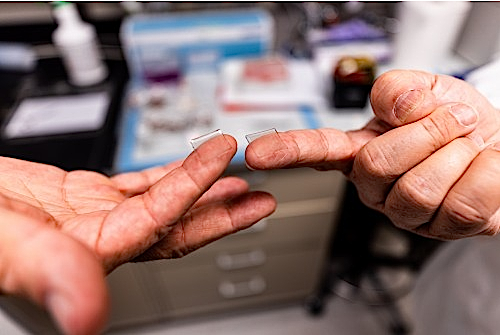
PittCo Vacc vaccine is delivered into skin through a fingertip-sized patch of microscopic needles.
CREDIT
Microneedle Array Vaccine.
|



